Effexor
Effexor (venlafaxine) was the first antidepressant in the class of drugs known as serotonin-norepinephrine reuptake inhibitors (SNRIs). It is available in an extended-release formula to treat depression, anxiety and panic disorders. It works by regulating levels of serotonin and norepinephrine in the brain.
- Medically reviewed by Jessica D. Hess, Ph.D.
- Last update: March 10, 2025
Effexor and Mental Health
The U.S. Food and Drug Administration approved Effexor in 1993 to treat major depressive disorder in adults. An extended-release version called Effexor XR won FDA approval in 1997. Both formulas contain the same active ingredient, venlafaxine.
Wyeth Pharmaceuticals Inc. manufactured both the original and extended-release versions until Pfizer acquired the company in 2009. Today, Pfizer only markets the extended-release version, which is also approved to treat generalized anxiety disorder, social anxiety disorder and panic disorder. The FDA approved the first generic version in 2010. Generic versions of the original formula are still available.
Venlafaxine is a serotonin-norepinephrine reuptake inhibitor, or SNRI. It helps block the reabsorption of both serotonin and norepinephrine, two chemicals in the brain that transmit nerve signals.
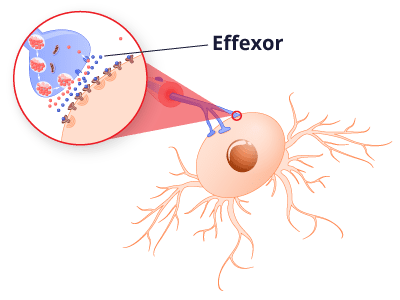
Scientists believe maintaining proper levels of serotonin in the brain prevents depression and anxiety, and they also think boosting norepinephrine reduces neuropathic pain.
Findings from one 2022 clinical research trial noted that high doses of venlafaxine are associated with changes in different forms of plasticity in discrete brain areas. In particular, the hippocampus plays a crucial role in venlafaxine-mediated antidepressant effects by promoting new nerve development or regulating the excitatory/inhibitory balance.
Research suggests venlafaxine may be more effective and cause fewer side effects than selective serotonin reuptake inhibitors, a slightly older class of antidepressants that focuses solely on serotonin. SSRIs include Prozac, Paxil and Zoloft.
Taking Effexor Safely
Pfizer discontinued the original version of Effexor in part because it required two or three doses per day while the extended-release version allows for a single daily dose for most patients. Suggested dosages for venlafaxine are significantly higher compared to many SSRI antidepressants. The capsules come in 37.5 mg, 75 mg and 150 mg strengths.



The medication guide instructs patients to take the drug with food at the same time each day. The medicine can make you sleepy, or make it harder for you to think clearly or react quickly. So, until you know how you will respond to the medication, it’s important to avoid driving, operating heavy machinery or doing other dangerous activities.
Interactions
Mixing venlafaxine with therapies that also affect serotonin levels, such as other antidepressants, linezolid, lithium, tramadol and St. John’s wort, can cause a condition called serotonin syndrome that raises blood pressure, heart rate and body temperature.
Let your doctor know of all prescription and over-the-counter medications and supplements you have taken or plan to take, including aspirin, migraine medications, amphetamines, antipsychotics and blood thinners.
Don’t start using Effexor XR within two weeks of taking a medication known as an MAOI, and don’t take an MAOI within a week of stopping venlafaxine.
Taking the medication with blood thinners or nonsteroidal anti-inflammatory drugs like Advil and Motrin may increase the risk of bleeding side effects. The medication guide also advises against drinking alcohol while taking the drug.
Recommended Dosages
Dosage and administration can be complicated and is detailed in a chart on the package insert. The recommended starting dose for major depressive disorder, generalized anxiety disorder and social phobia is 75 mg per day, but doctors may instruct some patients to start with 37.5 mg to allow their body to adjust to the medication.
The recommended starting dose for patients with panic disorder is 37.5 mg per day. Depending on need and how a patient tolerates venlafaxine, a doctor may increase the dose to 75 mg per day and then up to 225 mg per day. This is accomplished using 75 mg increments at intervals of no less than four days.
The maximum recommended dose for moderately depressed patients is 225 mg per day, but patients with severe depression may respond better to higher doses. One study referenced in the drug’s label found severely depressed patients responded to an average dose of 350 mg per day. But it’s unknown whether such patients need that high of a dose, and the label notes experience with venlafaxine doses higher than 225 mg per day is “very limited.”
Adjusting the Dose for Kidney or Liver Problems
The dose a doctor prescribes is unique to each patient. Do not change your dosage without consulting your doctor or pharmacist. The medication guide recommends doctors reduce the total daily dose by 50% in patients with mild to moderate liver failure, also known as hepatic impairment. It may be necessary to reduce the dose even more than 50% in some patients.
A reduced dose is also recommended for patients with kidney problems. The medication guide says doctors should reduce the dose by 25% for patients with a history of kidney failure and by 50% for patients undergoing hemodialysis.
Stopping Treatment
It’s unclear how long patients with depression, generalized anxiety disorder, social phobia or panic disorders should continue to take Effexor. During clinical studies for depression, the drug was effective for up to 52 weeks without having to adjust the dose. The drug’s label advises doctors to periodically reassess the need for the medication.
Never stop taking venlafaxine without a doctor’s direction. Abruptly stopping the drug or decreasing the dose too quickly can cause serious withdrawal symptoms, including extreme fatigue, confusion, dizziness, headaches and shock-like electrical sensations.
Side Effects and Warnings
Among the most unwanted effects of venlafaxine are those related to sexual dysfunction such as lack of interest in sex, difficulty becoming aroused and the inability to achieve orgasm. Other reactions commonly reported in clinical trials include nausea, dry mouth, constipation and sweating.
The more serious Effexor side effects, however, involve pregnant or nursing women, children, teens and young adults. Venlafaxine is not approved for use in pediatric patients, and the medication guide warns pregnant or nursing women not to take the drug without discussing the risks with their doctor.
Newborns whose mothers took the drug in the third trimester of pregnancy may experience serious problems right after delivery, including seizures and problems feeding and breathing. The drug may also pass through breast milk and harm a nursing baby.
Still, the drug’s strongest warning is an FDA boxed warning for suicidal thoughts and behaviors in patients under the age of 25.
Off-Label Uses
Although the FDA approved Effexor XR to treat four illnesses, doctors may prescribe the drug for other conditions. These unapproved treatments are called off-label uses.
A 2017 Canadian study published in the British Medical Journal looked at adults who visited certain physicians between January 2003 and September 2015 and were prescribed an antidepressant through an electronic prescribing system.
Researchers found nearly a third of the 106,850 antidepressant prescriptions written for 20,920 patients were for off-label uses. Researchers also determined only about 16% of off-label uses were backed up by medical research.
The researchers found SNRIs such as venlafaxine were less likely to be prescribed for off-label uses. But Effexor has been used to treat conditions for which it has never been approved.
Diabetic Neuropathy
A 2010 review published by American Family Physician called SNRI antidepressants such as venlafaxine “a promising category of antidepressants for treatment of diabetic peripheral neuropathic pain.” It cited a 2004 study in the journal Pain that found high doses of venlafaxine significantly reduced pain in diabetic neuropathy patients.
It also pointed to a 2007 Cochrane review that looked at three small studies on treating diabetic neuropathy with the drug. It found venlafaxine’s effectiveness at reducing pain was similar to that of tricyclic antidepressants such as amoxapine or desipramine.
“[SNRIs] are a promising category of antidepressants for treatment of diabetic peripheral neuropathic pain.”
However, a 2015 Cochrane review analyzed six trials involving neuropathic pain in general. It questioned the limits of the studies and cited considerable risk of bias in them.
“We found little compelling evidence to support the use of venlafaxine in neuropathic pain,” the researchers wrote in their conclusion.
Migraines
Effexor is also prescribed off-label to treat debilitating migraines. In April 2012, a neurologist from the Jefferson Headache Center at Thomas Jefferson University in Philadelphia presented new migraine treatment guidelines at the annual meeting of the American Academy of Neurology that formalized the drug’s role. The guidelines were published in the journal Neurology.
Hot Flashes
Venlafaxine has also been used for the off-label treatment of hot flashes associated with menopause. A study published in the Journal of the American Medical Association in 2006 found women who took the drug reported 60% fewer hot flashes. The study found the drug worked best at high doses, which meant an increased risk of side effects. Women should carefully weigh those risks against the benefits.
Calling this number connects you with a Drugwatch.com representative. We will direct you to one of our trusted legal partners for a free case review.
Drugwatch.com's trusted legal partners support the organization's mission to keep people safe from dangerous drugs and medical devices. For more information, visit our partners page.


