Cymbalta Lawsuits
People who say they suffered severe withdrawal symptoms after stopping Cymbalta (duloxetine) have filed lawsuits against its maker, Eli Lilly and Company (Lilly). In 2020, after a series of federal district court rulings in which the court denied plaintiffs’ attempts to seek class action status, plaintiffs’ cases were ultimately dismissed by the U.S. Court of Appeals for the Ninth Circuit.
- Legally reviewed by Julie Lawson Timmer, Esquire
- Last update: March 11, 2025
Cymbalta Class Action Certification
In October 2012, former users of the antidepressant Cymbalta filed a putative class action in the U.S. District Court for the Central District of California. The claim asserted that they, and other persons similarly situated, were harmed because the drug’s maker, Lilly, failed to warn about the drug’s withdrawal risks.
A class action is a type of legal action in which plaintiffs join together to sue as a “class” because they have similar claims against a common defendant or defendants. For any case to proceed as a class action, the court must certify the class. A “putative” class action refers to an action filed by a group of plaintiffs who believe their claims are related, but who have not yet received certification as a class.
In August 2013, a group of plaintiffs (a putative class) filed a motion in the federal district court in California, seeking certification of classes of consumers in four states: California, Massachusetts, Missouri and New York.
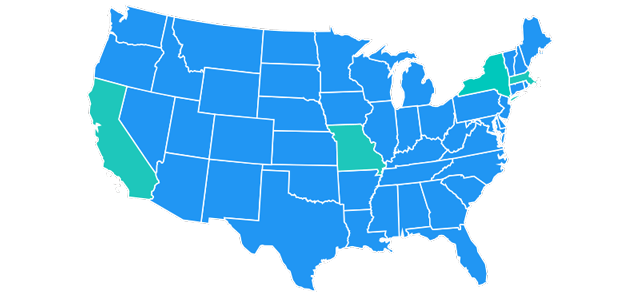
However, the district court denied the plaintiffs’ motions for class certification on Dec. 18, 2014.
Plaintiffs appealed to the Ninth Circuit U.S. Court of Appeals in June 2016. Their case was put on hold while the U.S. Supreme Court decided an unrelated case with similar legal issues involving Microsoft. When the Microsoft decision was issued in June 2017, Lilly pushed to have the putative class action against it dismissed based on the Microsoft ruling, which changed the law regarding class actions in a way that worked against the Cymbalta plaintiffs.
The Ninth Circuit agreed with Lilly and dismissed the putative class action in October 2017. As of June 2025, there have been no new developments. Drugwatch’s legal partners are currently not taking Cymbalta lawsuit cases.
A Timeline of Cymbalta Lawsuits and Settlements
Even before Cymbalta received approval from the U.S. Food and Drug Administration (FDA), Lilly was looking at a potential lawsuit over its use. Key Cymbalta dates include:
-
2020
The Ninth Circuit court denies the plaintiffs’ appeal of the district court’s refusal to reopen their cases, and shortly after, plaintiffs filed a petition for rehearing, which was denied.
-
2018
Plaintiffs filed another appeal to reopen the cases in federal district court. That court denied plaintiffs’ motion, and plaintiffs appealed again to the Ninth Circuit.
-
2017
Eli Lilly moves to dismiss the Strafford et al. v. Eli Lilly & Co. appeal, and in October 2017, the Ninth Circuit court dismisses the suit on jurisdictional grounds based on the U.S. Supreme Court’s Microsoft ruling. Lilly reports it has “reached a settlement framework” in 140 pending Cymbalta lawsuits. The Ninth 9th Circuit considers plaintiffs’ second appeal in Cymbalta class-action lawsuit.
-
October 2015
The Judicial Panel on Multidistrict Litigation (JPML) denies plaintiffs’ second request for a Cymbalta MDL. Plaintiffs appeal to the U.S. Court of Appeals for the Ninth Circuit.
-
August 2015
Lilly records first of four verdicts in its favor in individual lawsuits over Cymbalta.
-
July 2015
Plaintiffs again request an MDL, this time including more than 40 lawsuits.
-
December 2014
The JPML denies plaintiffs' request for an MDL. The panel found, among other things, that most of the common discovery had already taken place and there was an overlap in plaintiffs’ counsel. There could be “informal coordination with respect to the remaining common discovery, as well as other pretrial matters,” making centralization of the cases unnecessary.
-
August 2014
Plaintiffs in 25 federal lawsuits across the country petitioned the Judicial Panel for Multidistrict Litigation (JPML) to centralize the cases into a single multidistrict litigation (MDL) in the U.S. District for the Central District of California.
-
December 2013
South Carolina federal court rules in favor of Eli Lilly in lawsuit claiming the company failed to warn of withdrawal symptoms. Cymbalta users seek class-action certification in California, Massachusetts, Missouri and New York
-
April 2013
Lilly settles lawsuit with Peter Schilf’s parents for undisclosed amount.
-
October 2012
Former Cymbalta users file class-action lawsuit in California federal court.
-
2005
Lilly agrees to an undisclosed settlement in Traci Johnson’s death before a lawsuit is filed.
-
December 2004
Shortly after being prescribed Cymbalta, 16-year-old Peter Schilf commits suicide on Christmas Eve. His parents file a lawsuit against Eli Lilly.
-
August 2004
FDA approves Cymbalta as a treatment for major depressive disorder.
-
February 2004
College student Traci Johnson commits suicide while taking part in Cymbalta clinical trial. FDA later concludes suicide was not related to Cymbalta.
Lawsuit Claims
Lawsuits against the pharmaceutical company, Eli Lilly, say Cymbalta users face “severe physiological and psychological symptoms when they attempt to stop” taking the drug, including dizziness, nausea, headache, fatigue, paresthesia, vomiting, irritability, nightmares, insomnia, diarrhea, anxiety, hyperhidrosis and vertigo.
Most people may have taken the drug to treat depression or anxiety. But there are other FDA approved Cymbalta uses. These include treating diabetic peripheral neuropathy, fibromyalgia and certain kinds of chronic pain.
- “Overstated the efficacy of Cymbalta” and “downplayed and/or failed to state the true withdrawal side effects associated with Cymbalta”
- Failed to properly warn patients about the risks and of the “frequency, severity, and/or duration of Cymbalta withdrawal”
- Benefitted from patients who started taking Cymbalta again (becoming physically dependent on the drug) to avoid terrible side effects
- Advertised the benefits of the drug, even those that were not proven
- Produced a defective drug
The lawsuits also say Eli Lilly deceived the public by including in the drug’s label that only 1% of users experienced withdrawal symptoms, when in reality studies show between 44% and 50% suffered from discontinuation problems.
Of those who had withdrawal effects, about half experienced moderate or persistent symptoms. And one in 10 experienced severe symptoms. The plaintiffs say “nowhere on Cymbalta’s label does it indicate the potential duration of withdrawal symptoms.”
People Who Filed Lawsuits
Men and women who have filed Cymbalta withdrawal lawsuits say had they known the truth about the frequency, severity and duration of Cymbalta withdrawal they would not have started taking Cymbalta.
Among the people who filed lawsuits against Eli Lilly are:
Jennifer Saavedra
Jennifer Saavedra filed suit in California against Eli Lilly after she tried to stop using Cymbalta and experienced brain zaps, body shaking and tunnel vision. It took her a year to recover. In the meantime, her life was disrupted. Her complaint claimed withdrawal symptoms were more prevalent than Lilly claimed and that the company misled the public. Saavedra’s case eventually joined settlement proceedings, according to court documents from 2017.
Melissa Strafford
Melissa Strafford also filed her lawsuit in California. She was forced to continue using Cymbalta to avoid debilitating side effects. After she finally stopped taking the drug, she suffered for months. Her lawsuit accused Lilly of deceiving the public about the risks of the drug. The Strafford case was part of the putative class action that was ultimately dismissed by the Ninth Circuit in 2017.
- Brain Zaps
- Nausea
- Suicidal thoughts
- Nightmares
- Dizziness
- Weight changes
Patients of all ages who are started on antidepressant therapy should be monitored appropriately and observed closely for clinical worsening, suicidality or unusual changes in behavior.
Multidistrict Litigation Denied
Counsel for plaintiffs in 25 federal court proceedings against Eli Lilly tried unsuccessfully to have the then-filed cases and an unspecified number of future cases coordinated into a federal multidistrict litigation (MDL) in the U.S. District Court for the Central District of California. The purpose of an MDL is to avoid duplication and inconsistencies in the pretrial procedures and rulings and to conserve the resources of the parties, their counsel and the courts.
In a December 2014 order denying the MDL transfer, the U.S. Judicial Panel on Multidistrict Litigation found, among other things, that most of the common discovery had already taken place and there was an overlap in plaintiffs’ counsel, so there could be “informal coordination with respect to the remaining common discovery, as well as other pretrial matters,” making centralization of the cases into an MDL unnecessary.
Plaintiffs in all actions alleged:
- They suffered a variety of withdrawal symptoms after discontinuing use of Cymbalta
- The label for the drug failed to adequately warn of the risks of such symptoms
- Eli Lilly’s promotional campaigns for the drug overstated its efficacy while understating, downplaying or failing altogether to state its withdrawal side effects
The panel said in its order that it had been informed of 21 additional related federal actions. Plaintiffs’ counsel subsequently filed a second petition seeking MDL consolidation. The panel denied the petition in October 2015.
California Cymbalta Trials
About 35 individual and multi-plaintiff cases were filed in California state court. Many of the cases have been consolidated in a California Judicial Council Coordination Proceeding in Los Angeles. The first individual product liability cases went to trial in August 2015 and resulted in verdicts in favor of Eli Lilly. Two of the plaintiffs filed notices of appeal.
Herrera v. Eli Lilly & Co.
Claudia Herrera’s case against Eli Lilly became the first of four cases regarding Cymbalta withdrawal symptoms to go to trial in federal court in California in August 2015.
According to Herrera’s lawsuit, she began using Cymbalta in 2006 to treat her anxiety. In 2012, Herrera’s doctor instructed her to slowly stop taking the drug. Herrera says she suffered electric-like “zaps,” anxiety, spasms and suicidal thoughts, among other withdrawal symptoms, as a result.
Herrera accused Eli Lilly of downplaying its warnings to make Cymbalta more marketable. The jury cleared Eli Lilly of liability. Herrera filed a notice of appeal after losing her trial.
Cymbalta New York Trial
California is not the only state that saw legal actions against Cymbalta’s maker. In its 2015 annual report, Eli Lilly said it was “named in approximately 140 lawsuits involving approximately 1,300 plaintiffs filed in various federal and state courts alleging injuries arising from discontinuation of treatment with Cymbalta.” News reports from that same year say that the company faced more than 5,000 Cymbalta withdrawal cases. One publicized case is out of New York.
McDowell v. Eli Lilly & Co.
Jesse McDowell filed his lawsuit in New York, claiming Cymbalta’s label was inaccurate. McDowell said he suffered “serious and life-threatening withdrawal symptoms” from Cymbalta. He argued that the drug’s label was misleading because it said the symptoms occurred in patients “at a rate of greater than or equal to 1%” when the actual rates were closer to 50%.
U.S. District Judge Robert W. Sweet dismissed the suit, ruling the label adequately warned doctors about possible withdrawal symptoms. The federal judge said the label’s language followed FDA regulations.
Suicide Settlement
Cymbalta was not on the market long before it became tied to the suicide of a 16-year-old boy. Peter Schilf began taking samples of the antidepressant given by his doctor in November 2004, just three months after the drug gained approval from the U.S. Food and Drug Administration for treatment of depression. A month later, Schilf fatally shot himself on Christmas Eve. The FDA required its strongest warning, a black box warning, about suicide be added to the drug’s label in 2005.
Suicidality and Antidepressant Drugs
Antidepressants increased the risk compared to placebo of suicidal thinking and behavior (suicidality) in children, adolescents and young adults in short-term studies of major depressive disorder and other psychiatric disorders. Anyone considering the use of Cymbalta or any other antidepressant in a child, adolescent or young adult must balance this risk with the clinical need.
Short-term studies did not show an increase in the risk of suicidality with antidepressants compared to placebo in adults beyond age 24; there was a reduction in risk with antidepressants compared to placebo in adults aged 65 and older.
Depression and certain other psychiatric disorders are themselves associated with increases in the risk of suicide. Families and caregivers should be advised of the need for close observation and communication with the prescriber. Cymbalta is not approved for use in pediatric patients.
Eli Lilly Reports ‘Settlement Structure’ for Cymbalta Lawsuits
In Eli Lilly’s 2018 annual report, the company reported it had reached a “settlement structure” in 140 Cymbalta lawsuits involving roughly 1,470 plaintiffs. The cases were pending in various state and federal courts and all involved patients who claimed injuries caused by discontinuing use of Cymbalta.
“We have reached a settlement framework which provides for a comprehensive resolution of nearly all of these personal injury claims, filed or unfiled, alleging injuries from discontinuing treatment with Cymbalta. There can be no assurances, however, that a final settlement will be reached.”
The cases included about 40 lawsuits combined in a California Judicial Council Coordination Proceeding in Los Angeles. The company reported that the first individual cases went to trial in 2015, and the company had won verdicts against four plaintiffs suing over Cymbalta.
Lilly reported that the company believed the lawsuits were without merit and was prepared to “defend against them vigorously” in reporting on the settlement plans.
Calling this number connects you with a Drugwatch.com representative. We will direct you to one of our trusted legal partners for a free case review.
Drugwatch.com's trusted legal partners support the organization's mission to keep people safe from dangerous drugs and medical devices. For more information, visit our partners page.


