Mirena
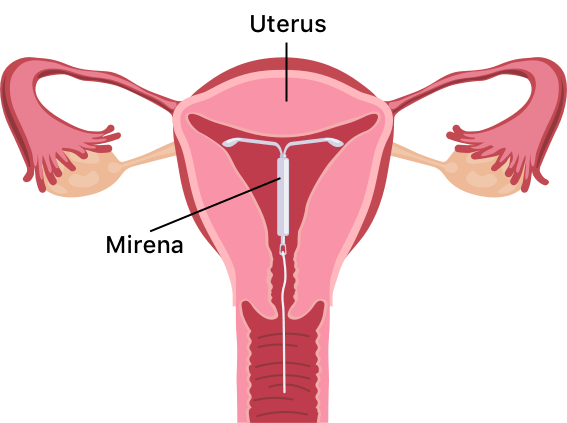
Mirena birth control is a T-shaped intrauterine device (IUD) from pharmaceutical manufacturer Bayer. The IUD releases levonorgestrel, a hormone that thickens the mucus in the cervix to stop egg fertilization and partially suppresses ovulation.
- Medically reviewed by Jessica D. Hess, Ph.D.
- Last update: June 6, 2025
What Is Mirena?
Mirena birth control is a T-shaped intrauterine device, or IUD, that releases the hormone levonorgestrel to thicken the mucus within the cervix, preventing the fertilization of eggs. The hormone also thins the lining of the uterus, which can partially suppress ovulation.
The Bayer-manufactured Mirena device is the #1 prescribed IUD in the US. It is made of soft plastic and measures 1.26 inches. A health care professional must place it in (and remove it from) your uterus. You will need to check its thin threads on the bottom yourself once inserted to ensure it is still in place.
Mirena contains a reservoir of levonorgestrel hormone. It lasts up to eight years for pregnancy prevention and eliminates the responsibility of taking a daily pill.
Levonorgestrel vs. Copper IUD
Long-acting, reversible contraception via IUD comes in two options: the hormonal or levonorgestrel IUD and the copper IUD. Both are very effective. It’s important to note that levonorgestrel is a progestogen vs. a progesterone.
The copper IUD has an efficacy rate of 99.2%, so out of every 1,000 people, eight will have an unintended pregnancy. The copper IUD does not contain hormones. It works because the properties of copper affect egg and sperm motility and survival.
A levonorgestrel IUD has a slightly higher efficacy rate of 99.8%, which means only two people out of every 1,000 will have an unintended pregnancy.
How Does Mirena Work?
Mirena birth control releases levonorgestrel, a type of progestin often used in birth control pills. The device slowly releases a continuous low dose of the hormone into your uterus and bloodstream.
Levonorgestrel thins the lining of the uterus and thickens the mucus in the cervix, making it difficult for sperm to move into and survive in the uterus. The thinning of the uterine lining also makes it less likely for an egg to attach to the uterus.
According to Bayer, Mirena is more than 99% effective at preventing pregnancy. The U.S. Food and Drug Administration approved using it for up to eight years.
Mirena Insertion and Removal
Your doctor can insert the Mirena device during a regular doctor’s visit. Mirena insertion takes about five minutes.
You should wait at least six weeks if you have just given birth, had a miscarriage or had an abortion.
You will have to get the Mirena IUD removed after eight years when it is no longer effective, but a doctor or other trained health provider can remove Mirena at any time. If the device moves out of place, your health care provider can insert a new one.
Mirena has no long-term effect on your ability to conceive. Eight out of 10 women who have had their Mirena IUD removed can get pregnant within a year.
Pros and Cons of Mirena IUD
Like any method of birth control, Mirena has pros and cons. On the positive side, it’s highly effective and begins working immediately. However, Mirena cannot work as emergency contraception.
“I will say there are times when Mirena is needed, and it is often a better choice than surgery or full-dose hormonal birth control.”
It’s so tiny you cannot feel it, nor can your partner. Mirena is very low maintenance; you don’t have to remember to do anything for it to function. And it is entirely and almost instantly reversible when you decide to get pregnant.
For some, one con is that a physician, OB-GYN or midwife must insert it. You may experience some cramping immediately after insertion. Additionally, the IUD does not protect against sexually transmitted diseases.
Benefits of Mirena
Mirena is the first and only hormone-releasing IUD that is FDA-approved to treat heavy periods for up to five years in women who choose an IUD for birth control.
Mirena thins the lining of the uterus, which can lessen heavy menstrual bleeding. In one clinical trial, blood loss was reduced more than 50% after six months in nine out of 10 women. It has also been shown to decrease menstrual pain and pain related to endometriosis.
A 2022 clinical study of 80 participants studied the effects of the Mirena IUD on endometrial thickness and curative effect in patients with perimenopausal abnormal uterine bleeding. The endometrial thickness and menstrual volume scores following three months of treatment were remarkably lower than those before treatment and were considerably lower than those of the control group.
- Mirena is more than 99% effective.
- You don’t have to worry about remembering to take a pill.
- It lasts up to eight years, according to the FDA.
- Most insurance plans cover Mirena.
- It is long-term and reversible birth control.
- Women can still breastfeed while using Mirena.
- It uses a lower dose of hormones than some other birth control methods.
- It reduces or stops menstrual flow in women who don’t want a period or suffer from excessive bleeding.
Experts also recommend IUDs such as Mirena as a birth control option for teens and younger adults because they are very effective, need no daily care and last for many years.
Disadvantages of Mirena
As with any birth control method, you should weigh the risks and benefits before using Mirena. One of the disadvantages of Mirena is that it may cause ovarian cysts.
Becoming pregnant while using Mirena can be life-threatening for the mother and baby under certain circumstances.
- It may cause ovarian cysts.
- Pregnancy with Mirena can be life-threatening.
- IUDs can become infected during insertion.
- Device insertion can cause perforation.
- It doesn’t protect against STDs.
- Devise expulsion can occur, leaving the woman at risk of pregnancy.
IUDs may lead to severe infection or pelvic inflammatory disease and perforation of the uterine wall or cervix can occur.
For more information, speak to your doctor about the risks of using Mirena.
Mirena Complications
Severe Mirena side effects are rare, but can potentially include a risk of breast cancer and intracranial hypertension or pseudotumor cerebri, an unexplained pressure inside the head that can lead to vision loss.
Upon removal, some women experience symptoms sometimes called “Mirena Crash.” The sudden reduction in hormone levels can cause symptoms such as mood swings, breast tenderness, nausea, sadness, or depression and flu-like symptoms.
Women who say the IUD injured them have filed thousands of Mirena lawsuits.
Who Should Not Use Mirena?
Women who are pregnant or suspect they might be pregnant should not use Mirena. There are several gynecological conditions that make the use of Mirena ill-advised or dangerous. These include uterine abnormalities such as fibroids, a history of pelvic inflammatory disease, postpartum endometriosis, a recent infected abortion, abnormal pap smears, or genital bleeding or infections.
Individuals with a history of liver disease or tumors, or breast cancer, should avoid using Mirena.
If you have known hypersensitivities to the IUD or any of its components, avoid Mirena and consider other birth control options.
Mirena Interactions
When you take two or more medicines at the same time, the effects of one medicine can counteract the other, which is known as an interaction. It’s important, therefore, to tell your doctor what medications you are taking.
Certain drugs and herbal supplements can make Mirena less effective. Examples of these substances include some blood thinners, anti-anxiety drugs and anti-seizure medications. Anti-retroviral drugs, antibiotics and St. John’s wort may also interact with Mirena.
How Much Does Mirena Cost?
Mirena can cost anywhere from $0 to $1,300. Under the Affordable Care Act, plans in the health insurance marketplace must cover some type of contraception.
Bayer has indicated that its surveys show 95% of women were covered for an IUD like Mirena, with little to no out-of-pocket costs.
If you do not have insurance, the cost of Mirena is $1,049.24, or $12.49 per month over a 7-year period.
Calling this number connects you with a Drugwatch.com representative. We will direct you to one of our trusted legal partners for a free case review.
Drugwatch.com's trusted legal partners support the organization's mission to keep people safe from dangerous drugs and medical devices. For more information, visit our partners page.



