Heater-Cooler Devices
Heater-cooler devices are machines used in almost all open-heart or open-chest bypass surgeries to regulate a patient’s body temperature. The most commonly used model of heater-coolers, LivaNova’s Stockert 3T, has been linked to the spread of bacteria called nontuberculous mycobacteria (NTM). The bacteria can cause serious chronic lung infections in immune-compromised patients and sometimes even results in death if left untreated.
Heater-cooler devices such as the Stockert 3T are necessary for use in certain surgeries to regulate a patient’s body temperature (to keep them from becoming too warm or too cold).
These devices are especially important for use in surgeries involving the heart and lungs (cardiothoracic surgeries), such as cardiopulmonary bypass procedures, which involve the intentional and temporary stopping of a patient’s heart and/or lung functioning.
In 2010, the U.S. Food and Drug Administration began receiving reports of patients developing serious infections. By 2015, the agency determined heater-cooler devices were responsible for the problem. And in 2016, the agency was able to link the infections specifically to the Stockert 3T.
In 2020, the FDA reiterated that LivaNova developed a design to reduce, but not eliminate, the risk of airborne transmission of non-tuberculosis mycobacterium (NTM) from the 3T device. And in 2022, FDA sent a warning letter to CardioQuip, LLC violations to Quality Systems Regulation at its facilities where it produces its Modular Cooler-Heater Model MCH-1000.
How Does a Heater-Cooler Device Work?
While heater-cooler devices fall under cardiovascular regulations, these surgical-assistive machines can be used in a variety of other medical procedures as well.
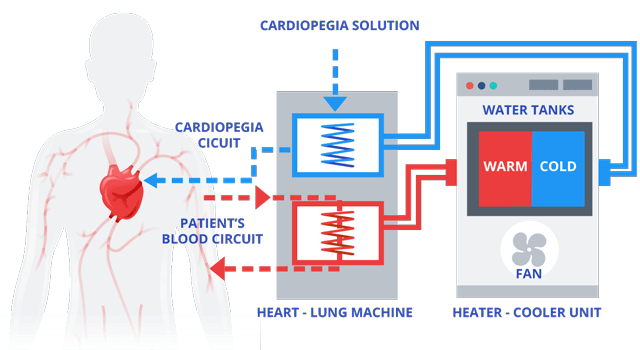
Heater-cooler devices include a water tank separating warm water from cold water as well as a heating and cooling unit that allows air to flow in and out. The devices provide temperature-controlled water to an external heat exchanger, such as a heart-lung machine, or to warming/cooling blankets through closed water circuits which have no contact with open air.
This mechanical process helps maintain an internal balance versus external changes in a patient’s body temperature.
The water in the heater-cooler device is isolated from the cardioplegia solution used to intentionally and temporarily stop the heart as well as the patient and the blood circuits.
Heater-Cooler Device Brands Used In Surgeries
Various manufacturers are responsible for the manufacturer and marketing of several different brands of heater-cooler devices used in surgeries. At the time of the infections, German manufacturer Sorin built the model most commonly used in the U.S., the Stockert 3T. In June 2015, Sorin merged with Cyberonics, Inc. and the new company was named LivaNova.
LivaNova’s Stockert 3T heater-cooler is used in about 60 percent of the 250,000 surgeries performed in the United States each year requiring a heater-cooler device.
- Maquet
- Heater-Cooler Unit HCU 40 (as well as older models)
- Medtronic, Inc.
- Biotherm Heat Exchanger and ECMOtherm II Heat Exchanger
- Alsius Corporation
- Thermoguard XP
- Jostra AB
- Jostra HCU 30
- Cincinnati Sub-Zero Products, Inc.
- Cincinnati Sub-Zero Hemotherm 400CE Dual Reservoir Cooler/Heater and CSZ Hemotherm 400MR
- Terumo (Sarns) Cardiovascular Group
- Terumo HX@ Heater Cooler; Terumo Sarns 11160 Heater Cooler and Terumo Sarns TCM II Heater Cooler
But it is the Stockert 3T that appeared to be at the root of a series of infections during a five year period beginning in 2010.
LivaNova’s Stockert 3T Linked to Bacterial Contamination
The U.S. Food and Drug Administration (FDA) approved the Stockert 3T in June 2006. The FDA classifies it and other heater-coolers as Class II devices. As defined by the agency, Class II devices “are higher risk than Class I and require greater regulatory controls” to ensure the device’s safety and effectiveness.
Between January 2010 and August 2015, the FDA received 32 Medical Device Reports (MDRs) of patient infections resulting from contaminated heater-cooler devices used during various open-heart or open-chest surgical procedures. The FDA received a total of 25 of those 32 reports in 2015 alone.
In 2015, the FDA issued a safety communication advising the public and health care practitioners of a certain serious, potentially fatal, form of bacteria associated with heater-cooler devices. The bacteria known as nontuberculous mycobacterium (NTM) are commonly found in the environment in our natural resources, such as water and soil. While the bacteria are generally not harmful, exposure can lead to infections in some patients.
The agency added that in some cases it “may cause infections in very ill patients and/or in individuals with compromised immune systems,” which would include the majority of patients undergoing open-chest surgical procedures.
Treatment can persist for months and even years; and if left untreated, patients infected by NTM can be at risk of death.
Sorin Group Recalls its Heater-Cooler, FDA Sends Warning Letter
In June 2015, Sorin Group, by then known as LivaNova, recalled its Stockert 3T Heater-Cooler devices due to “potential colonization of organisms, including mycobacteria.” The recall noted that the potential is greater if devices are not properly disinfected and maintenance is not performed per the instructions for Use.
Potentially affected devices were distributed worldwide and nationwide in the U.S., reaching nearly 100 various consumer markets around the globe.
The FDA advised patients that in the U.S. most cardiopulmonary bypasses include the use of a heater-cooler device. It was further found that the affected devices may have been in distribution up until 2014, with the first incident of infection being reported as early as 2012.
The FDA issued a Warning Letter to LivaNova in December 2015 after inspecting its facilities at two different locations, one in Germany and one in the U.S. The letter stated that during the FDA’s inspection of LivaNova’s German facility investigators determined the “firm’s devices are adulterated” in methods used for manufacturing, packing, storing or installation. The FDA found that such practices were “not in conformity with the current good manufacturing practice requirements of the Quality System regulation.”
At the conclusion of the inspection, the FDA noted that as a part of the company’s recall, LivaNova issued an update to its cleaning and disinfection instructions following complaints of patient deaths due to infections caused by the Stockert 3T. But the FDA concluded that the acceptance criteria for the test did not demonstrate that the updated procedures produced a reduction in bacteria.
The FDA said that it would remain “actively engaged” in the process.
Study Finds Contamination Originated in Factory
Over two years following the recall, in mid-July 2017, a study published in The Lancet found traces of two major M. chimaera species of nontuberculous mycobacteria (NTM) in LivaNova’s German-based factory. Scientists matched those samples found in the factory where the Stockert 3T heater-cooler units are manufactured with sample NTM bacteria in open-chest surgery patients in the U.S., as well as sampling from heater-cooler units used in multiple hospitals.
The study determined that contamination of heater-cooler units at the LivaNova factory “seems a likely source for cardiothoracic surgery-related severe M. chimaera infections diagnosed in Switzerland, Germany, the Netherlands, the UK, the US and Australia.”
Those patients diagnosed worldwide since 2013, included more than 100 severe cases of NTM infections, some of which resulted in death.
Types of Surgeries that Use the Stockert 3T Heater-Cooler
Cardiothoracic surgeries involve any surgical procedure having to do with the heart, lungs, esophagus and other organs in the chest. Thoracic surgeries – having to do with the part of the body between the neck and the abdomen, primarily the chest – are usually performed to treat the lungs, esophagus and chest wall. Cardiac, or heart, surgeries are performed to treat the heart, heart valves and arteries in the heart and chest.
It has been estimated that more than 500,000 people had surgeries that involved the Stockert 3T heater-cooler during the time NTM contamination was linked to the devices.
- Lung cancer
- Benign diseases and tumors of the lung
- Chest reconstruction
- Esophageal (esophagus) cancer
- Esophageal reconstruction
- Benign tumors in the esophagus
- Gastroesophageal reflux (digestive disease in which stomach acid irritates the lining of the esophagus)
- Mesothelioma
- Pleural (lung membranes) diseases
- Chest wall tumors
- Excessive sweating
- Heart transplant
- Lung transplant
- Insertion of stent for narrowing airways
- Angioplasty (surgical repair or unblocking of a blood vessel)
- Artificial heart valve surgery (to replace a damaged heart valve with a functioning one)
- Atherectomy (to remove atherosclerosis – build-up of fats, cholesterol and other substances – from blood vessels)
- Bypass surgery (taking arteries or veins from other parts of the body –grafts – and using them to reroute blood around a blocked artery)
- Insertion of stents to open arteries, improve blood flow or relieve chest pain (angina)
- Cardiomyoplasty (healthy muscle from another part of the body is wrapped around the heart)
Heater-Cooler Device Complications
In some cases, patients became aware of the infections months or even years following their surgeries. The FDA noted that for this reason, it is possible that not all cases have been reported. In 2016, the Washington Post reported that “more than a half-million patients could have been exposed to the bacteria.”
The FDA issued its first safety communication regarding the problem in 2015, advising the public of a link between the use of heater-cooler devices and contracted NTM infections. The agency pointed out that the majority of patients acquiring the infection had undergone cardiothoracic surgical procedures.
Contamination from Heater-Cooler Devices
Although the water in the Stockert T3 never comes into direct contact with the patient, there is the potential for a contaminated water source to enter other parts of the device or to transmit the bacteria through the air. The possibility of airborne bacteria exposure is due to air leaving the device’s exhaust vent, which can filter into the environment and the patient during a surgical procedure. This is called aerosolization, which is the process of converting a physical substance into the form of particles able to be carried in the air (aerosol).
In 2016, the Centers for Disease Control and Prevention (CDC) alerted hospitals and patients of the potential for exposure to NTM for patients who underwent open-heart or open-chest surgeries involving the Stockert 3T heater-cooler device going back to January 1, 2012.
The CDC said in its health alert that “new information” indicated that the devices manufactured by LivaNova were contaminated with the bacteria during manufacturing.
Some factors that may increase the potential for aerosolization of NTM bacteria include:
- Water agitation or bubbling inside the tank via pumps (heart-lung machines)
- Mixing components
- Return circuit water
- Laminar (constant streamline) flow disruption
- The location and the positioning of the device’s exhaust fan may come into play here
- Air filters
- Some heater-cooler devices have them and some do not; but most are likely unable to capture NTM bacteria
- Water filters
- Not all devices have these either; but these should be able to remove most NTM from tap water used in the device
- Fans
- These are on most devices and may facilitate the movement of aerosolized NTM from the device into the operating room
Calling this number connects you with a Drugwatch.com representative. We will direct you to one of our trusted legal partners for a free case review.
Drugwatch.com's trusted legal partners support the organization's mission to keep people safe from dangerous drugs and medical devices. For more information, visit our partners page.

