Transvaginal Mesh Lawsuit
People filing transvaginal mesh lawsuits report complications, including pain, bleeding and organ perforation. One of the largest transvaginal mesh settlements to date was $830 million for 20,000 cases in multidistrict litigation (MDL). Although the MDL closed in November 2022, women can still file lawsuits in state courts.
- Last update: July 3, 2025
What is the Transvaginal Mesh Lawsuit?
Transvaginal mesh lawsuits, also known as pelvic mesh lawsuits, claim that several manufacturers of transvaginal mesh knew that their products were defective and could cause complications such as erosion, organ perforation, severe pain, painful sex, mesh migration and other serious issues that require surgery to remove the mesh. The term “transvaginal” refers to the technique surgeons used to implant the mesh through the vagina, rather than through the abdomen. The theory was that it would be a less invasive form of surgery.
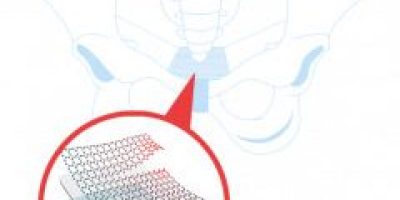
Pelvic mesh is made of polypropylene woven plastic and serves as a hammock to support weakened or damaged tissue. It’s used to treat pelvic organ prolapse (POP), a condition where organs sag drop from their normal position and push against the vaginal walls. These prolapses occur for different reasons but generally because of a weakened pelvic floor due to aging, childbirth, weight gain or other reasons. These organs include the bladder, bowel, uterus and rectum. Pelvic Mesh is also used to treat stress urinary incontinence, or SUI, a condition where urine leaks when pressure is placed on the bladder, such as during laughing, coughing, sneezing or exercise. Doctors surgically implant mesh through the vagina, and it acts like a net to hold up organs.
Because of the health risks, the FDA banned transvaginal mesh for POP in 2019. Most women who filed transvaginal mesh lawsuits received the mesh for POP or SUI. Some of these women required multiple surgeries to remove the mesh, and even after multiple surgeries, pieces of the mesh remained and continued to cause discomfort and problems. The main claims are that manufacturers produced faulty products and failed to warn the public of the health risks.
“After [transvaginal mesh surgery] I was in terrible pain. My pelvic area was on fire. Sex was out of the question because it hurt so bad. I was getting urinary tract infections (UTIs) on a regular basis.”
Latest Transvaginal Mesh Lawsuit Updates
As of July 2025, lawyers continue to take individual transvaginal mesh lawsuits to pursue settlement or trial even after the original seven multidistrict litigations have closed. Pending lawsuits continue in state courts across the country, though the exact number of cases is unknown.
According to the New York Times, settlements and verdicts awarded to injured plaintiffs totaled close to $8 billion in 2019. Since then, the exact totals for settlements have been difficult to calculate because some cases have been resolved confidentiality. Cases involved pelvic mesh used to treat pelvic organ prolapse or stress urinary incontinence.
Transvaginal Mesh Litigation Highlights
-
July 2025
Individual transvaginal mesh lawsuits continue to advance and sometimes receive settlements at the state level. A trial involving Ethicon is scheduled to begin on July 21 in New Jersey. Lawyers are still accepting new cases from those who have been impacted.
-
March 2025
While there have been no major updates in the litigation to report on over the last few months, transvaginal mesh lawsuits remain active in state courts. Lawyers are continuing to investigate and accept new lawsuits.
-
September 2024
Our research into court documents showed that a handful of Ethicon cases in New Jersey were resolved or dismissed. Judge Padovano also ordered the closing of the Mazie Slater Bard Qualified Settlement Fund, which originally opened in November 2020 to disburse settlement funds to a select group of plaintiffs. The exact criteria for how this group was chosen was not public record.
-
August 2024
New Jersey Judge Gregg A. Padovano ordered that cases not subject to the Ethicon Master Settlement Agreement proceed with discovery, according to our review of Case Management Order #113. Four new pelvic mesh cases against Ethicon joined the litigation in New Jersey and discovery will begin. About 140 women in England reached an undisclosed settlement with Johnson & Johnson, Boston Scientific and Bard for transvaginal mesh injuries.
-
June 2024
The Eleventh Circuit Court of Appeals upheld a win this month for a woman who claimed that the Coloplast vaginal mesh implanted in her body was defective. She had been awarded a $2.5 million verdict that will now stand.
-
May 2024
Plaintiffs, such as Regina Oesterle and her husband Benjamin Oesterle, continue to file pelvic mesh claims in state courts. Oesterle’s lawsuit 1:23-cv-11848-JEK in Massachusetts State Court concerns Boston Scientific’s Obtryx bladder mesh sling. The judge has set deadlines throughout the year, with a status conference in December 2024. Status conferences are a chance for the parties to touch base on litigation progress and for the judge to weigh in on any matters that require their attention. If the parties have nothing to discuss, the judge may cancel or reschedule the conference.
-
March 2024
Most mesh cases filed have been resolved in settlement or dismissal. But lawyers continue to accept transvaginal mesh cases and file lawsuits in state courts across the country.
-
January 2024
Judge Gregg A. Padovano, the judge overseeing pelvic mesh cases in New Jersey, extended the discovery deadline for all Bard mesh cases in New Jersey to April 30, 2025 to give the parties more time to prepare cases for potential trials.
-
December 2023
Lawyers continue to pursue new vaginal mesh lawsuits with mixed results since the MDL closed.
-
May 2023
A New Jersey jury found in favor of Johnson & Johnson in Rebecca Dandy’s suit against the company’s subsidiary Ethicon. Dandy claimed she suffered injuries after using Ethicon’s TVT-O Prolene mesh sling.
-
February 2023
The U.S. Supreme Court upheld a $302 million judgment against Johnson & Johnson in a case where the state of California accused the company of concealing the risks of its mesh products. Provided the case doesn’t go to appeal again, J&J and the State of California will agree on how and when the funds will be disbursed. This information is not always made public.
-
November 2022
The last MDL closed, and U.S. District Judge Joseph Goodwin of the Southern District of West Virginia remanded cases back to their respective state courts.
-
July 2022
According to a court filing, 95% of all transvaginal mesh cases have been resolved or dismissed.
-
March 2021
Boston Scientific agreed to pay $189 million to settle claims with 47 states that it deceptively marketed its mesh devices to consumers.
Some patients have won multimillion-dollar verdicts against manufacturers. One jury, for example, awarded $68 million to Mary McGinnis and her husband in 2018. Another jury awarded Patricia Mesigian $80 million in 2019.
At one time, over 100,000 lawsuits had been filed in the MDLs against Johnson & Johnson’s Ethicon unit, Boston Scientific, American Medical and Bard.

Why People Filed Transvaginal Mesh Lawsuits
Women who filed lawsuits after receiving the pelvic mesh devices for prolapse or stress urinary incontinence (SUI) say they suffered from painful injuries such as chronic pain, vaginal erosion and mesh migration.
- Erosion
- This occurs when implants damage vaginal walls or internal organs.
- Infection
- Bacteria growth on implants can cause infections.
- Pain
- The device may damage nerves, cut through tissues or shrink, causing scarring and pain — including painful intercourse.
- Urinary problems
- The devices may block the bladder, making it difficult to urinate.
- Recurring prolapse
- Even after mesh surgery, the implant can fail and cause recurring prolapse.
- Recurring incontinence
- Some women experience new or worsened incontinence of the bladder or rectum.
These women had problems such as sitting, walking, having sex and participating in other activities, according to lawsuits. The complications were so bad that many women had to suffer through multiple revision surgeries to remove the implants.
“I expected everything to be fine after the operation, but I have been in constant pain since that day.”
Pelvic Mesh Plaintiff Profile: Regina Oesterle v. Boston Scientific Corporation
Regina Oesterle filed her pelvic mesh lawsuit against Boston Scientific in 2023 in the United States Court for the District of Massachusetts, Case 1:23-cv-11848-AK.
This case is still ongoing, and the judge extended deadlines for discovery and depositions throughout 2025 at the request of lawyers on both sides because it was taking longer than anticipated to gather medical records. According to the lawyers, extending deadlines will not damage the legal rights of any parties in the case.
Oesterle had transvaginal surgery in July 2020. Her surgeon implanted the Boston Scientific Obtryx Transobturator Mid-Urethral Sling System to treat stress urinary incontinence.
Pain, disfigurement, unnecessary medical expense, embarrassment and harm.
Compensation for economic and non-economic losses, such as disfigurement, pain, mental anguish and emotional distress.
Allegations Against Transvaginal Mesh Manufacturers
Women who filed lawsuits claim that “Pelvic Mesh Products had not been adequately tested and found to be safe and effective for the treatment of incontinence and prolapse.” Furthermore, defendants provided patients with false and misleading information about how safe and effective the products supposedly were.
- Intentionally misleading the U.S. Food and Drug Administration, the medical community, patients and the public about the true safety and effectiveness of the products.
- Failing to properly test devices.
- Failure to research the risks of the products.
- Failing to create safe and effective methods to remove the materials.
- Failing to adequately warn people of potential complications and injuries.
The products were cleared for use based on “weak evidence,” according to a 2017 study in BMJ.
Many women were never warned about the risks. Donna Miser told Drugwatch she received a bladder sling for urinary incontinence and suffered complications such as pain during sex and knife-like pain in her vagina. When doctors examined her, the mesh had eroded and embedded itself in her urethra, bladder and vaginal walls. She had one surgery to remove the mesh and still needs to have more.
“I’ve utilized the ‘crisis line’ a couple of times … because I became suicidal. When the pain hits a ’10,’ I become a whole different person. I am slowly watching my life disappear,” Miser said.
Finding a Transvaginal Mesh Lawyer
Once you’ve worked with your surgeon to address any issues caused by transvaginal mesh, the second step is to speak with an attorney to learn about your legal options. Look for a transvaginal mesh attorney who has a background in personal injury lawsuits and product liability law.
Even though the MDL for transvaginal mesh closed in November 2022, it may be wise to find a lawyer who has handled these cases in multidistrict litigation. Transvaginal mesh lawyers are still taking cases despite the closure of the MDL.
You want to find an attorney you feel comfortable talking to, and one with a track record of securing compensation for victims of transvaginal mesh. Some plaintiffs prefer to work with a female lawyer because these cases require communication about female anatomy and sensitive topics. Make sure to ask how many mesh cases the attorney has handled and the outcomes of those cases.
Drugwatch’s legal partners offer free consultations, and it’s easy to sign up by clicking on the free case review buttons on this page. Once you sign up, an attorney will get back to you right away and ask you about your experience with pelvic mesh. Take this opportunity to ask questions about what to expect, about the firm’s experience and about how they can help you.
Settlements and Verdicts
So far, manufacturers have paid out billions in transvaginal mesh settlements and jury verdicts. Most recently, the Eleventh Circuit Court of Appeals upheld Virginia Redding’s $2.5 million jury verdict against Coloplast in June 2024.
In February 2023, Johnson & Johnson’s subsidiary Ethicon agreed to pay a nearly $10 million settlement to the state of Kentucky to resolve claims that the company engaged in deceptive marketing of pelvic mesh devices.
-
JANUARY 2014
Coloplast settled 400 lawsuits
-
APRIL 2014
AMS settled 20,000 claims
-
JULY 2014
Bard settled 500 lawsuits
-
MARCH 2015
Ethicon (Johnson & Johnson) settled one lawsuit for $5 million before trial
-
APRIL 2015
Boston Scientific settled 3,000 claims for $119 million
-
AUGUST 2015
Bard settled another 3,000 claims for about $200 million
- January 2016
-
DECEMBER 2017
Boston Scientific settled about 350 claims
-
MARCH 2021
Boston Scientific agreed to pay $188.7 million to settle deceptive marketing claims by 47 states and Washington, D.C.
Lawsuit Verdicts
J&J, Bard and Boston Scientific each lost multiple bellwether trials. The first major mesh verdict came in the case of Christine Scott in July 2012. A California jury awarded her $5.5 million against C.R. Bard. Since then, several verdicts have been awarded in favor of plaintiffs.
Some companies, such as Boston Scientific and Bard, settled cases before trial for undisclosed amounts.
- Dunfee vs. Ethicon
- In June 2019, jurors awarded Linda Dunfee $500,000 in her case against Ethicon. This was the ninth time Philadelphia jurors found in favor of plaintiffs against Ethicon, according to Law360. Dunfee received Prolift mesh for POP in 2007. Nearly two years later, the device had eroded through the vaginal wall.
- Mesigian v. Ethicon
- In May 2019, Patricia Mesigian received a jury award of $80 million in her lawsuits against J&J’s Ethicon. The jury allotted $50 million for punitive damages and $30 million for compensatory damages — the highest compensatory damage award for mesh injury at the time, Bloomberg reported. Mesigian received Ethicon’s Prolift mesh for POP in 2008. She suffered from pain, inflammation, infection and scar tissue. She had multiple surgeries to try and remove the mesh to correct complications, but complete removal was impossible, and she continued to suffer complications.
- McFarland v. Ethicon
- Susan McFarland won a $120 million verdict against Ethicon in April 2019, the Philadelphia Inquirer reported. McFarland had Ethicon’s TVT-O device implanted for stress urinary incontinence in 2008. McFarland suffered pain and constant infections, and removing the mesh didn’t alleviate symptoms. The Philadelphia jury awarded $100 million for punitive damages — damages awarded to discourage the defendant from especially negligent behavior.
- McGinnis v. C.R. Bard
- A New Jersey jury awarded Mary and Thomas McGinnis a total of $68 million in April 2018 in their lawsuit against C.R. Bard. Mary McGinnis had been injured after being implanted with an Avaulta Solo and an Align Transobturator. The award to the Raleigh, N.C. couple consisted of $33 in compensatory damages and $35 million in punitive damages. "I'm very grateful," Mary McGinnis said as she left the courtroom with her husband, according to northjersey.com. "This case was fought for all the victims of mesh, whom I hold in my heart." In 2023, the New Jersey Supreme Court ruled that Bard didn’t get a fair trial, though there was no mention of whether a new trial will be scheduled or if the verdict will be thrown out, Law.com reported.
- Blankenship, Campbell, Tyree and Wilson v. Boston Scientific
- Jeanie Blankenship, Carol Sue Campbell, Jacquelyn Tyree and Chris Rene Wilson had each been implanted with the Boston Scientific Obtryx product. In 2014, a New Jersey jury held Boston Scientific responsible for the women’s medical bills and suffering. The jury awarded “$250,000 and punitive damages of $1,000,000 to each plaintiff. Additionally, the jury awarded future-compensatory damages of $3 million to the first plaintiff, $3 million to the second, $3.5 million to the third, and $4 million to the fourth,” according to the Fourth Circuit Court of Appeals. The verdict was upheld in February 2018.
- DuBois-Jean, Dotres, Nunez and Betancourt v. Boston Scientific
- Margarette DuBois-Jean, Margarita Dotres, Mania Nunez and Juana Betancourt all claimed Boston Scientific had been negligent in Pinnacle mesh’s defective design. A Florida jury awarded each of the women more than $6 million in November 2014, but Boston Scientific appealed. In October 2017, the 11th Circuit Court of Appeals upheld the $26.7 million verdict.
- Hammons v. Ethicon
- Patricia Hammons won a more than $12.5 million award after she sued J&J for injuries one of its products caused. Hammons’ Prolift implant failed, and she had multiple revision surgeries. The jury awarded Hammons $5.5 million for her injuries and $7 million in punitive damages. A state appeals court upheld the verdict in June 2018.
- Engleman v. Ethicon
- Peggy Engleman won a $20 million jury verdict after she said Ethicon’s TVT-Secur device caused serious complications that the company did not warn her about. The device caused pain, bleeding and infections that required multiple surgeries to fix, her lawsuit said.
- Hyrmoc v. Ethicon
- Elizabeth Hrymoc was awarded $4 million for pain and suffering and $10 million for punitive damages against J&J’s Ethicon subsidiary. The New Jersey jury also awarded $1 million to Hrymoc’s husband for loss of conjugal affection. Hrymoc received Ethicon’s Prolift and transvaginal tape for incontinence and had several corrective surgeries for pain and other complications.
- Ebaugh v. Ethicon
- In September 2017, a jury awarded Ella Ebaugh $57.1 million in her case against Ethicon. “It was a very happy day, but I was still sad and depressed,” Ebaugh told CBS 3 Philly. “I’m in excruciating pain when I’m standing, it hurts when I’m sitting.”
- Beltz v. Ethicon
- Sharon Beltz won $2.4 million in a trial against Ethicon in May 2017 after she said the company’s Prolift mesh caused complications. The company appealed and in August 2019, the Supreme Court of Philadelphia upheld her verdict.
- Carlino v. Ethicon
- Ethicon lost its trial against Sharon Carlino and the jury awarded the woman $13.7 million. Carlino successfully defended J&J’s appeal in April 2019 and the verdict was upheld.
- Gross v. Ethicon
- Linda Gross had Ethicon’s Prolift implanted in 2006 for POP. Gross experienced excruciating pain that resulted in anxiety, depression and other complications. In February 2013, a New Jersey jury awarded her $3.35 million in compensatory damages and $7.76 million in punitive damages.
Transvaginal Mesh Insurance Class Action
In 2015, an insurance company for the device maker Caldera Medical Inc. filed a class-action suit against its client over a coverage dispute. Caldera faced 2,184 claims from women who alleged injuries from the products when Federal Insurance Co. took the company to court.
On March 3, 2017, U.S. District Judge Stephen Wilson approved the settlement in the amount of $12.25 million. “The settlement is made in good faith; and is fair, reasonable, adequate, and consistent with due process,” Wilson wrote in his order.
The case is Federal Insurance Company v. Caldera Medical, Inc. and the case number is 2:15-CV-00393. While the deadline to submit a class action claim was May 2, 2016, you may still be able to file an individual claim. Contact a transvaginal mesh lawyer to learn more about your options.
Transvaginal Mesh Lawsuit Frequently Asked Questions
Calling this number connects you with a Drugwatch.com representative. We will direct you to one of our trusted legal partners for a free case review.
Drugwatch.com's trusted legal partners support the organization's mission to keep people safe from dangerous drugs and medical devices. For more information, visit our partners page.