Actemra
Actemra (tocilizumab) is a biologic medication for adults with moderate to severe rheumatoid arthritis that did not respond well enough to methotrxate or another disease-modifying antirheumatic drug (DMARD). Doctors also prescribe the immunosuppressive drug to treat giant cell arthritis in adults and polyarticular juvenile idiopathic arthritis (PJIA) and systemic juvenile idiopathic arthritis (SJIA) in patients 2 years old and older.
- Medically reviewed by Mireille Hobeika, Pharm.D.
- Last update: June 12, 2025
Actemra changes the way the immune system works. It comes as intravenous infusions and/or subcutaneous injections in single-dose prefilled syringes or autoinjectors, depending on the condition it’s treating.
The Food and Drug Administration first approved tocilizumab in 2010. Genentech, a subsidiary of Roche, makes the medication.
In its October 2018 Investor Update, Roche reported the drug had brought in the equivalent to nearly $1.6 billion worldwide in the first nine months of 2018. The United States was the drug’s biggest market, and Americans spent about $620 million on tocilizumab prescriptions.
-
JANUARY 2010 — Rheumatoid Arthritis
The FDA approved tocilizumab for adults who have moderate to severe rheumatoid arthritis and have tried one or more disease-modifying antirheumatic drugs (DMARDs) without success.
-
APRIL 2011— Systemic Juvenile Idiopathic Arthritis
The FDA expanded the drug’s uses to include treatment of patients who are 2 years old or older and have active systemic juvenile idiopathic arthritis, a chronic inflammatory joint disease that affects children who are under 16 years old.
-
APRIL 2013 — Polyarticular Juvenile Idiopathic Arthritis
The FDA added polyarticular juvenile idiopathic arthritis treatment to the list of approved uses but limited the use to patients who are 2 years old or older. This type of arthritis affects five or more joints in children under 16 years old.
-
MAY 2017 — Giant Cell Arteritis Treatment
The FDA approved tocilizumab to treat adults who have giant cell arteritis, a severe inflammatory disease of blood vessels that mostly affects people who are over 50 years old.
-
AUGUST 2017 — CAR T Cell-Induced Cytokine Release Syndrome
The FDA again expanded the drug’s uses, this time to include severe or life-threatening cytokine release syndrome caused by chimeric antigen receptor (CAR) T cell therapy, an immunotherapy designed for the treatment of certain cancers.
How Actemra Works
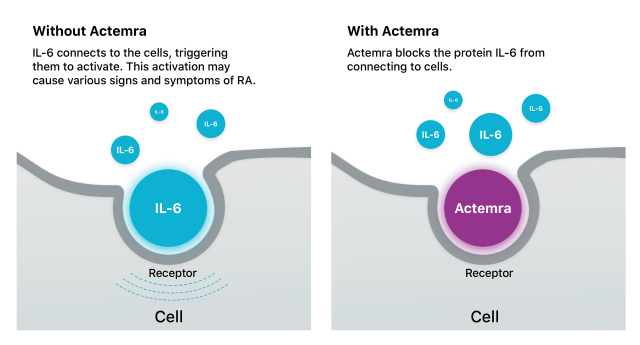
Actemra works by blocking the action of a certain protein in the body called interleukin 6 (IL-6). IL-6 acts as both a pro-inflammatory and anti-inflammatory. It’s said to have a role in rheumatoid arthritis and its effects.
IL-6 connects to the body’s immune cells and triggers them to activate, which may cause various signs and symptoms of RA. Tocilizumab is the first RA drug to block IL-6 from connecting to cells.
Patients can receive the medication via two different methods: subcutaneous injection or intravenous (IV) infusion.
Subcutaneous injection is an injection under the skin. The medication comes in a single-use, prefilled syringe or in a single-use, prefilled autoinjector called ACTPen. The FDA approved the pen in November 2018.
Patients can inject themselves at home, or a caregiver or health care professional can do it for them. The recommended sites for self-injection are the front of the thigh or the abdomen except for the 2-inch area around the belly button. For each new injection, choose a different injection site that’s at least 1 inch from the last area where you injected the medicine.
Patients receive the IV form at a health care provider’s office or other medical center. A medical professional administers the liquid solution into the vein with a needle. This type of infusion takes about one hour.
Dosing depends on a person’s weight, how the drug is given and the condition it’s treating. Other factors can also influence the recommended dosage. These include any pre-existing health conditions and whether the patient takes other medications.
Recommended Dosages
- Rheumatoid Arthritis
- IV infusion: 4 mg per kg given once every four weeks; the dose may be increased to 8 mg per kg once every four weeks, depending on the patient’s tolerance and on the improvement of symptoms; dosing should not exceed 800 mg per IV infusion
Subcutaneous injection: 162 mg once every other week in patients who weigh less than 220 pounds and 162 mg once every week in patients who weigh over 220 pounds - Giant Cell Arteritis
- IV infusion: Not approved for GCA
Subcutaneous injection: 162 mg every week - Polyarticular Juvenile Idiopathic Arthritis
- IV infusion: 8 mg per kg for patients at or above 30 kg and 10 mg per kg for patients less than 30 kg every four weeks as a 60-minute single intravenous drip infusion
Subcutaneous injection: 162 mg every three weeks in patients less than 30 kg and 162 mg every two weeks in patients at or above 30 kg - Systemic Juvenile Idiopathic Arthritis
- IV infusion: 8 mg per kg for patients at or above 30 kg and 12 mg per kg for patients less than 30 kg every two weeks as a 60-minute single intravenous drip infusion
Subcutaneous injection: 162 mg every week in patients at or above 30 kg and 162 mg every 2 weeks in patients less than 30 kg - Cytokine Release Syndrome
- IV infusion: 8 mg per kg for patients at or above 30 kg and 12 mg per kg for patients less than 30 kg as a 60-minute single intravenous drip infusion; if there is no improvement in the symptoms after the first dose, up to three additional doses can be given with an interval of at least eight hours between consecutive doses; dosing should not exceed 800 mg per IV infusion
Subcutaneous injection: Not approved for CRS
Side Effects
Actemra side effects can be mild or severe. Common side effects include upper respiratory tract infections, headache, increased blood pressure and reactions at the injection site.
The drug has a black box warning — the FDA’s most serious warning — for the risk of serious infections that may lead to hospitalization or death. These include tuberculosis, invasive fungal infections, viral infections and bacterial infections. People who take tocilizumab with methotrexate or corticosteroids are most at risk.
“Actemra changes the way your immune system works. This can make you more likely to get infections or make any current infection worse. Some people have died from these infections,” according to the Actemra website.
“Actemra changes the way your immune system works. This can make you more likely to get infections or make any current infection worse. Some people have died from these infections.”
In addition, people who have taken the drug have suffered stomach tears, changes in blood test results and reactivation of a dormant hepatitis B virus. Though rare, some patients may suffer multiple sclerosis, and the drug can increase your risk of certain cancers, according to the drug’s website.
The FDA has also received reports of serious or deadly side effects that are not listed on the drug’s label. Reported issues include heart failure, heart attack and stroke.
Before You Take Tocilizumab
Some patients should not take Actemra, so it’s important to talk with your doctor about your medical history before you start taking the drug.
- Active or chronic infection
- Tuberculosis or a history of TB
- Liver disease
- Diverticulitis
- Stomach ulcer
- Stomach or intestinal bleeding
- Diabetes
- HIV or a weak immune system
- Hepatitis B
- Cancer
- Disorders affecting the nerves such as multiple sclerosis
- Low count of certain blood cells (low platelet count and/or low neutrophil count)
- High cholesterol levels and/or high triglycerides levels
Women should also let their doctors know if they are pregnant or plan to become pregnant. Animal data suggests the drug may cause harm to unborn babies, but studies of the drug in pregnant women are too limited to determine whether the drug can cause birth defects and miscarriages in humans.
A pregnancy exposure registry monitors pregnancy outcomes in women who take the drug while they are pregnant. Genentech encourages physicians and pregnant women to register. The company advises against taking the drug and breastfeeding.
Interactions
Before starting tocilizumab, provide your doctor with a list of all other substances you use. This includes medications, supplements and alcohol.
Using Actemra with other drugs or substances can change the way the drug works in the body. Drug interactions can increase or decrease the effectiveness of tocilizumab or the other drugs or substances you use.
- CYP450 Substrates
- Certain drugs, including oral contraceptives and cholesterol lowering drugs, are metabolized or broken down by liver enzymes such as the CYP450 enzymes before the drugs are eliminated from the body. By decreasing inflammation, tocilizumab increases the activity of the CYP450 enzymes, which leads to a faster break down of these drugs. So these drugs are eliminated from the body faster and thus are less effective. Patients who are taking tocilizumab in conjunction with drugs like oral contraceptives and cholesterol lowering drugs should exercise caution as the drugs' effectiveness may be decreased. The effect of tocilizumab on the activity of the CYP450 enzymes, and therefore on the effectiveness of the drugs metabolized by these enzymes, can last for several weeks after stopping treatment.
- Alcohol
- Limit the amount of alcohol you drink while taking the medication. This can help prevent further injury to the liver. Tocilizumab is linked to elevated liver enzymes and liver toxicity. Doctors should assess patients for liver injury before starting treatment
- Live Vaccines
- Avoid live vaccines if you take tocilizumab. Researchers haven’t evaluated how safe and effective live vaccines are with the drug. Patients should be up-to-date on vaccines before starting treatment. Let your doctor know if you are scheduled to receive a live vaccine such as the shingles vaccine, the chickenpox vaccine or the MMR vaccine.
Actemra Versus Humira and Enbrel
Other FDA-approved rheumatoid arthritis medications include Humira and Enbrel. The labels for these medications warn of serious side effects that are not included in the label for Actemra. This has led many patients and doctors to see tocilizumab as a safer choice.
But studies and reports to the FDA have revealed the three drugs carry similar risks. In fact, a 2017 STAT investigation showed the risk of certain side effects is as high or higher with Actemra compared to other rheumatoid arthritis medications.
The drugs are similarly effective in treating rheumatoid arthritis. They treat other conditions as well.
| CATEGORY | ACTEMRA | HUMIRA | ENBREL |
|---|---|---|---|
| Manufacturer | Genentech | AbbVie | Amgen |
| FDA Approval Date | 2010 | 2002 | 1998 |
| Average Cost for RA | About $31,877 a year * | About $41,460 wholesale cost per year ** | About $41,468 wholesale cost per year ** |
| Dose for RA | IV: 4 mg per kg of weight every four weeks; subcutaneous injection: 162 mg weekly or every other week depending on weight | 40 mg every other week | 50 mg weekly; pediatric doses range from 0.8 mg per kg of weight to 50 mg weekly, depending on the patient's weight |
| Most Common Side Effects | Infections, common cold, headache, high blood pressure, injection site reactions and increased ALT (liver enzymes) | Infections, injection site reactions, headache and rash | Infection and injection site reactions |
Calling this number connects you with a Drugwatch.com representative. We will direct you to one of our trusted legal partners for a free case review.
Drugwatch.com's trusted legal partners support the organization's mission to keep people safe from dangerous drugs and medical devices. For more information, visit our partners page.