Abbott Laboratories
Abbott Laboratories is a U.S. health care company. It specializes in cardiovascular, diagnostics, diabetes and neuromodulation products. Abbott also produces nutrition brands including Pedialyte, Ensure, Glucerna and Similac. In 2022, Abbott recalled Similac baby formula for potential Cronobacter contamination.
About Abbott
Abbott Laboratories is a U.S. health care company, well-known for its medical devices and nutritional products. Its best-selling products are its cardiovascular and neuromodulation products, followed by nutritional products, diagnostics and pharmaceutical drugs.
Abbott Laboratories has over 130 years’ experience in the consumer health care and pharmaceutical industry. The company has 31 manufacturing sites and 12 development centers around the world.
Abbott employs about 113,000 people in over 160 countries. Its headquarters is in Chicago, Illinois at Abbott Park. As of 2022, Robert B. Ford is Abbott Laboratories’ current CEO.
Abbott History
Abbott got its start in 1888. Practicing physician and drug store owner Wallace Abbott founded Abbott Alkaloidal Company.
Abbott made about $2,000 in its first year. The company was incorporated in 1894.
In 1907, Abbott expanded its reach outside the U.S. with the opening of an office in London, England. Abbott produced its first synthetic medicine, Chlorazene, in 1916. This antiseptic medicine was used to treat wounded World War I soldiers.

Abbott’s breakthroughs and acquisitions continued into the 21st century. Abbott officially separated from its sister company, AbbVie, in 2013.
Abbott wanted to focus on heart stents and other medical devices. AbbVie’s main concentration is biologic drugs.
Abbott acquired St. Jude Medical and Alere Inc. in 2017. It paid more than $28 billion for both companies. Abbott wanted to expand its reach and boost its diagnostics presence in the U.S. and worldwide.
Abbott Products
Abbott is the manufacturer of many popular products in the therapeutic areas of cardiovascular, diabetes, diagnostics, neuromodulation, nutrition and pharmaceuticals.
Some of its well-known brands include Similac, PediaSure, Pedialyte, Brufen, Klacid, Alinity, Ensure, FreeStyle, iStat and MitraClip.
The company claims to be a market leader in blood screening, adult and pediatric nutrition, left ventricular assist devices, remote heart failure monitoring, and point-of-care testing.
Abbott Vascular, Medical Devices and Drugs
- Amplatzer Steerable Delivery Sheath: A cardiac catheter
- Assurity MRI Pacemaker: Magnetic resonance (MR) conditional pacemaker
- HeartMate 3 Left Ventricular Assist Device (LVAD): Assists the heart’s left ventricle in pumping blood throughout the body and increasing oxygen supply to organs
- MitraClip Transcatheter Mitral Valve Repair System: World’s first transcatheter mitral valve repair to be used as a last-resort therapeutic option
- FreeStyle Family: Blood glucose monitoring systems
- Alere i: Detection system to differentiate between influenza virus A and B
- Alinity: Point-of-care and diagnostics systems including blood testing
- BurstDR Stimulation: Neurostimulation to treat chronic pain
- Jude Medical Infinity DBS System: Deep brain stimulation therapy
- Xience: Coronary (vascular) stents
- Biaxin/Klacid: Macrolide antibiotic (clarithromycin)
- Brufen: Non-steroidal anti-inflammatory drug (NSAID/ibuprofen)
- Creon: Replaces digestive/pancreatic enzymes to improve food digestion
- Dicetel: Calcium channel blocker to treat irritable bowel syndrome (IBS)
- Femoston: Hormone replacement therapy (HRT) drug for women
- Influvac: Seasonal flu vaccine
- Luvox: Antidepressant used to treat obsessive-compulsive disorder (OCD)
- Synthroid: Thyroid hormone
- Tarka/Teveten: High blood pressure medications
- Tricor: Reduces cholesterol and fatty acids (triglycerides) in the blood
Abbott Lawsuits and Settlements
Abbott has faced lawsuits over its drug Tricor, FreeStyle diabetes products and St. Jude defibrillators. The company was also named co-defendant alongside AbbVie in lawsuits over the popular pharmaceutical drugs Humira and Depakote.
Amplatzer Steerable Delivery Sheath Lawsuits
Abbott issued an Amplatzer Steerable Delivery Sheath recall in June 2023, and patients began filing ASDS lawsuits after they suffered from complications such as air embolisms.
The device maker recalled the device because of a design problem that led to an increase in ASDS complications such as air embolisms. Air embolisms can lead to stoke, low blood pressure, irregular heart rate and reduced flow of blood to the heart. ASDS lawsuits claim the device is defective and Abbott failed to warn patients and medical providers of the risk.
These lawsuits are still in early stages, and there have been no court-approved global settlements or jury verdicts.
Similac Baby Formula Lawsuits
In recent years, parents of premature infants have filed Similac lawsuits against Abbott. Lawsuits say Abbott knew that Similac increased the risk of NEC, or necrotizing enterocolitis, a serious gastrointestinal problem that may be fatal.
Studies have found a link between cow’s milk baby formula and NEC in premature infants. Mead Johnson, maker of Enfamil, is also named in baby formula lawsuits. As of 2022, litigation is in early stages and there have been no settlements or jury verdicts.
Our Trusted Legal Partners
Drugwatch partners with trusted law firms to help you take legal action. After submitting the form, one of Drugwatch's partners will contact you for a free case review.



St. Jude Class Action Lawsuit
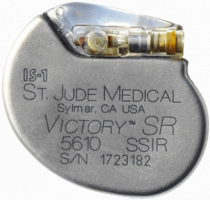
Just months after acquiring St. Jude Medical, Abbott found itself mixed up in a nationwide class action suit.
The lawsuit claims that St. Jude Medical knew about a battery-depletion defect in some of its cardiac defibrillators starting in 2011, but the company did not adequately report the risk.
Instead, the company waited almost five years before initiating a recall of the faulty devices in October 2016.
The class action suit was filed on behalf of public and private health insurance payers who had to pay for the high-priced defective devices and surgeries. Plaintiffs are seeking just over $9.9 million in damages.
FreeStyle Diabetes Products Lawsuits
The parents of a diabetic woman who died after using Abbott’s FreeStyle test strips and blood glucose monitor system sued Abbott.
They filed the wrongful death lawsuit in Cook County Circuit Court in Chicago, Illinois, in December 2014.
Abbie Harper died in November 2013. Abbott recalled the test strips Harper was using four days after her death.
Harper’s parents alleged that the defective test strips provided inaccurately low blood glucose results. This caused Harper to receive too little insulin from her insulin pump.
Harper got her prescription for the FreeStyle test strips filled at a Walgreens store. Walgreens was also named in the lawsuit.
Harper went two days with receiving too little insulin before she died from diabetes-related complications.
Depakote Lawsuits

Depakote lawsuits claim children suffered birth defects because their mothers took Depakote during pregnancy. Abbott rid itself of all rights and responsibilities for Depakote when it separated from AbbVie.
Tricor Antitrust Class Action Settlement
In late-2008, Abbott agreed to pay $184 million to settle antitrust claims brought by competitor pharmaceutical companies, drug stores and other purchasers.
Competitors who sued Abbott included Teva Pharmaceuticals and Impax Laboratories. They claimed Abbott blocked cheaper generic versions of its cholesterol medication Tricor from making their way to consumers.
Abbott reached another settlement in April 2009 to resolve the last of the claims. That settlement cost Abbott another $250 million. Tricor brought in more than $1 billion in sales for Abbott in 2007.
Humira Lawsuits

Humira lawsuits blame the drug for causing infection and cancer. Patients have sued Abbott and AbbVie over the drug.
Abbott Recalls
Abbott recalled some of its popular medical devices and other products after they caused patient injuries and deaths.
-
2022
Product Recalled – Similac powdered baby formula Reason for Recall – reports of babies becoming ill with Cronobacter infections after ingesting Similac from the company’s Michigan factory, some babies died
-
2019
Product Recalled – implantable cardioverter defibrillators
Reason for Recall – electrical failures due to a faulty manufacturing process causing Aluminum wires to be exposed
Number of Units Affected – 108 in the U.S. -
2018
Product Recalled – HeartMate 3 Left Ventricular Assist System
Reason for Recall – malfunction in the device that can slow down or stop pump flow, setting off a persistent low flow alarm in the system and causing blood clots and death
Number of Units Affected – 4,878 in the U.S. -
2018
Product Recalled – implantable defibrillators
Reason for Recall – potential for hacking
Number of Units Affected – 350,000 -
2017
Product Recalled – Abbott pacemakers including Accent, Anthem, Accent MRI, Accent ST, Assurity and Allure (St. Jude implantable defibrillators)
Reason for Recall – potential for hackers to take over the devices
Number of Units Affected – 465,000 -
2017
Product Recalled – three different coronary catheters including NC Trek RX Coronary Dilatation Catheter and NC Traveler coronary dilatation catheters and NC Tenku RX PTCA balloon catheters
Reason for Recall – 19 reports of injury and one report of death due to difficulty removing the protective balloon sheath; defect resulted in problems with inflating or deflating the balloon during procedures; it caused air embolism, thrombosis (clot in the artery), heart attack and death
Number of Units Affected – 132,040 in the U.S. -
2014
Product Recalled – FreeStyle Flash Blood Glucose Monitoring System and FreeStyle Blood Glucose Monitoring System
Reason for Recall – potential to produce low blood glucose results when used with older FreeStyle test strips
Units Affected – expands on of November 2013 recall of 20 specific lots of FreeStyle and FreeStyle Lite blood glucose test strips in U.S.
Calling this number connects you with a Drugwatch.com representative. We will direct you to one of our trusted legal partners for a free case review.
Drugwatch.com's trusted legal partners support the organization's mission to keep people safe from dangerous drugs and medical devices. For more information, visit our partners page.


