Januvia & Janumet
Januvia and Janumet are prescription drugs that belong to a group of Type 2 diabetes drugs called incretin therapies. These drugs work by signaling the pancreas to create more insulin and decreasing the sugar made by the liver. They come in several different dosages.
- Medically reviewed by Patricia Hartke, Pharm.D., BCPS
- Last update: March 13, 2025
Januvia (sitagliptin) is an oral Type 2 diabetes medication manufactured by Merck & Co. The U.S. Food and Drug Administration (FDA) approved the drug in 2006, and it is one of the most popular Type 2 diabetes drugs on the market. In 2007, the FDA approved a variation of Januvia called Janumet, which is a combination of sitagliptin and metformin. Janumet also comes in an extended release formula called Janumet XR. In 2017, the FDA approved another variation of Januvia called Steglujan, which is a combination of sitagliptin and ertugliflozin.
Both Januvia and Janumet belong to a class of drugs called dipeptidyl peptidase 4 (DPP-4) inhibitors that work by helping the body produce more insulin. Januvia was the first DPP-4 approved by the FDA. Januvia and Janumet will likely bring in multi-billion sales in the near future. Merck stands to benefit from the patent on the drug until 2022.
In clinical trials Januvia proved effective in controlling blood sugar levels. However, some studies reported rare and serious side effects, including acute pancreatitis, severe joint pain, and, possibly, pancreatic cancer. However, recent information does not clearly link sitagliptin to pancreatic cancer.
How Do Januvia and Janumet Work?
Januvia helps lower blood sugar in two ways. It helps the body increase insulin to stabilize blood sugar and decrease sugars that are made in the liver. It is a part of the class of diabetes medications called DPP-4 inhibitors. DPP-4 is a protein made by the body that plays a role in glucose metabolism.
The process works like this: After a person eats and blood sugar rises, intestinal cells release hormones called incretin hormones. Incretin hormones stimulate pancreatic cells called beta cells to release insulin to metabolize sugar and signals the liver to stop making excess sugar. In people without diabetes, DPP-4 breaks down incretin hormones to keep blood sugar and insulin levels balanced. For people with diabetes, too much sugar is already in the blood.
These drugs block DPP-4, allowing incretin hormones to stay in the blood longer and to continue stimulating the pancreas into make more insulin to remove excess sugar.
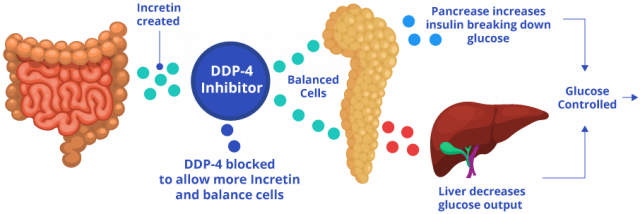
Janumet is similar to Januvia, except it also contains metformin. So, in addition to stimulating the body to produce more insulin like Januvia does, the metformin in Janumet decreases the glucose produced by the liver and decreases the absorption of glucose by the intestines.
How Effective are Januvia and Janumet?
About 5,200 people participated in the initial clinical trials for Januvia. The majority of study participants were white and the average age was 55 years. Both Januvia and Janumet reduced blood sugar in clinical trials.
An A1C (glucose level in the blood) measurement of 7 percent or lower is ideal for most people with Type 2 diabetes. In one study with 1,262 patients who took Januvia for 18 weeks or 24 weeks, researchers found 100 mg daily lowered A1C by 0.7 percent and 0.8 percent respectively.
Januvia also reduced fasting plasma glucose (FPG) by about 13 mg/dL in people who started with an average FPG of 180 mg/dL in the 18-week study. Januvia reduced FPG by about 12 mg/dL in people who started with an average FPG of 170 mg/dL in the 24-week study. A normal FPG is 100 or lower.
In clinical studies for Janumet, researchers did not actually use Janumet. They obtained their data by administering sitagliptin and metformin. They found using both drugs lowered A1C and FPG more than using either drug alone.
A1C levels dropped about 1.4 percent in patients with an average starting A1C of 8.8 percent who took 50 mg sitagliptin and 500 mg of metformin twice daily. It dropped 1.9 percent in those with an average starting A1C of 8.8 percent who took 50 mg sitagliptin and 1000 mg metformin twice daily.
Side Effects
In clinical trials, researchers reported the most common side effects that occurred in 5 percent or more of patients taking Januvia include nasopharyngitis (common cold), respiratory tract infections and headaches. Januvia may also come with more rare but serious side effects.
Patients should consult their doctor immediately if they experience any serious Januvia side effects. This is not a complete list of side effects. Always speak to a doctor or health care provider for more information.
- Nausea
- Diarrhea
- Swelling of hands or legs
- Headache
- Upper respiratory infection
- Stuffy or runny nose
- Sore throat
- Pancreatitis (swelling of the pancreas that can cause vomiting, fast heart rate, nausea, stomach and back pain)
- Allergic reactions (hives, difficulty breathing, swelling of the lips, face, tongue or throat)
- Heart failure (fast increase in weight, shortness of breath, or swelling of the feet)
- Severe skin reaction (rash, blistering or peeling, burning in eyes, swelling of the face)
- Hypoglycemia (low blood sugar)
- Fatal and nonfatal necrotizing pancreatitis (tissue in the pancreas dies and causes an infection, usually a more severe form of acute pancreatitis)
- Severe joint pain
Janumet Side Effects and Black Box Warning
Because Janumet is a combination medication, it has the side effect risks of Januvia plus metformin. In people who took Janumet, the most common side effects that occurred in 5 percent or more of clinical trial participants include diarrhea, headache and respiratory infections. More people who took Janumet instead of Januvia experienced low blood sugar (hypoglycemia) and gastrointestinal issues such as gas, indigestion and abdominal discomfort.
Because Janumet is made with metformin, it carries a black box warning for lactic acidosis. This condition occurs when lactic acid accumulates in the bloodstream. Symptoms of lactic acidosis include feeling cold in hands and feet, dizziness, irregular heartbeat, weakness and nausea. People who suffer from lactic acidosis should seek medical care immediately.
FDA Warnings and Post-Market Safety Studies
The most serious side effects associated with Januvia and Janumet are acute pancreatitis and severe joint pain.
In 2009, the FDA received 88 reports of acute pancreatitis linked to Januvia and Janumet — some of which were fatal. In March 2013, the agency announced it was gathering more data on Januvia’s link to pancreatitis and possible pancreatic cancer. In 2014, the FDA said it there was no cause for concern with safety risks in its review of reports linking Januvia and other DPP-4’s to pancreatitis or pancreatic cancer. But the agency said its review was ongoing.
In 2015, the FDA warned that Januvia and Janumet could cause severe joint pain. The time of onset of the pain, which can be disabling, can vary from just one day to years after beginning drug treatment. Some patients experienced a recurrence of symptoms after the stopping the drug and restarting it, or starting a different drug in the same class of DPP-4 inhibitors.
Dosage Strengths and Recommendations
Januvia and Janumet come in a variety of dosages, depending on each individual’s needs for glucose control. These drugs are not meant to treat people with Type 1 diabetes. People who have excess toxins known as ketones in the blood or urine — a life-threatening condition known as diabetic ketoacidosis — should not take Januvia or Janumet.
Januvia Dosages
The recommended starting dose of Januvia is 100 mg once daily with or without food. Depending on the patient, the prescribing health care provider may adjust the dose. People with a history of serious allergic reactions to sitagliptin should not take Januvia.
Because the kidneys filter Januvia out of the body, people whose kidneys are not fully functioning may need a lower dose of Januvia. The recommended dose for people with moderate kidney impairment is 50 mg and 25 mg for patients with severe kidney impairment or end-stage kidney disease that requires dialysis. Health care providers should test a patient’s kidney function before starting Januvia.



Janumet Dosages
Janumet dosages are customized for each patient, but should not exceed 100 mg sitagliptin/2000 mg metformin. The recommended starting dose is 50 mg sitagliptin/500 mg metformin. Patients should take Janumet twice daily with meals, and dose increases should be gradual to minimize gastrointestinal side effects from metformin.
If patients are already taking metformin, doctors should continue them on that dose plus 50 mg sitagliptin. Patients already taking 850 mg of metformin should take 50 mg sitagliptin/1000 mg metformin.
Like Januvia, the proper functioning kidneys are necessary to filter Janumet out of the body. Patients with severe kidney problems should not take Janumet.


Drug Interactions
According to the medication inserts of Januvia and Janumet, there are few drug interactions. But these drugs were not tested with every drug that could react with them.
Patients should use caution when taking Januvia or Janumet with medications known to cause hypoglycemia, or abnormally low blood sugar levels, such as a sulfonylurea and insulin.
- Digoxin (Januvia)
- Alcohol may increase risk of lactic acidosis (Janumet)
- Carbonic anhydrase inhibitors such as topiramate, zonisamide, acetazolamide or dichlorphenamide may increase risk of lactic acidosis (Janumet)
- Insulin may increase risk of low blood sugar (Januvia and Janumet)
Calling this number connects you with a Drugwatch.com representative. We will direct you to one of our trusted legal partners for a free case review.
Drugwatch.com's trusted legal partners support the organization's mission to keep people safe from dangerous drugs and medical devices. For more information, visit our partners page.


