Avandia
Avandia (rosiglitazone) has common, mild side effects, such as headaches and cold-like symptoms. But it also has links to serious side effects, including heart attacks. Avandia has a black box warning for congestive heart failure.
Our content is developed and backed by respected legal, medical and scientific experts. More than 30 contributors, including product liability attorneys and board-certified physicians, have reviewed our website to ensure it’s medically sound and legally accurate.
legal help when you need it most.
Drugwatch has provided people injured by harmful drugs and devices with reliable answers and experienced legal help since 2009. Brought to you by The Wilson Firm LLP, we've pursued justice for more than 20,000 families and secured $324 million in settlements and verdicts against negligent manufacturers.
More than 30 contributors, including mass tort attorneys and board-certified doctors, have reviewed our website and added their unique perspectives to ensure you get the most updated and highest quality information.
Drugwatch.com is AACI-certified as a trusted medical content website and is produced by lawyers, a patient advocate and award-winning journalists whose affiliations include the American Bar Association and the American Medical Writers Association.
About Drugwatch.com
- 15+ Years of Advocacy
- $324 Million Recovered for Clients
- 20,000 Families Helped
- A+ BBB Rating
- 4.9 Stars from Google Reviews
Testimonials
I found Drugwatch to be very helpful with finding the right lawyers. We had the opportunity to share our story as well, so that more people can be aware of NEC. We are forever grateful for them.
- Medically reviewed by Jessica Elste, Pharm.D., BCPS
- Last update: November 3, 2025
- Est. Read Time: 8 min read
What Is Avandia?
Avandia is the brand name for rosiglitazone, a prescription medication used to manage blood sugar in adults with Type 2 diabetes. It works by improving the body’s sensitivity to insulin, which helps lower blood glucose levels.
Avandia is part of the thiazolidinedione (TZD) class of drugs, which is the same class as Actos (pioglitazone). The pharmaceutical company GlaxoSmithKline (GSK) originally developed and marketed Avandia, which received FDA approval in 1999. Its usage in the U.S. peaked around 2006.
What Are the Most Common Side Effects of Avandia?
The most common side effects of Avandia in clinical trials were cold-like symptoms and headaches. Avandia has a few other mild side effects that may last for a short time.
These mild side effects rarely require medical treatment.
- Fatigue and tiredness
- Headache
- Upper respiratory tract infections (such as colds)
- Weight gain
Mild, common Avandia side effects are less severe than the major risks listed below, but they can still impact your quality of life. If they bother you or don’t go away, make sure to tell your doctor.
Serious Health Risks Linked To Avandia
Avandia’s most serious side effects, such as congestive heart failure and heart attacks, affect the heart. These side effects are rarer but also more serious and may require emergency medical care.
- Congestive heart failure
- Heart attack (myocardial infarction)
- Bone fractures (hand, arm or foot)
- Liver problems or failure
- Macular edema (swelling behind the eye)
Older adults and those with preexisting heart conditions are at a significantly higher risk of heart complications.
Bone fracture risk is higher in women and in people who have used Avandia for over a year.
Avandia has a black box warning for congestive heart failure, and the drug can cause new heart failure or make preexisting heart failure worse. Black box warnings are the most serious safety alerts issued by the U.S. Food and Drug Administration (FDA).
Latest Side Effects Information for Avandia
As of October 2025, heart attack, congestive heart failure, stroke and coronary artery disease were the most common serious Avandia side effects reported to the FDA. These four conditions account for 89.4% of all Avandia adverse events in the FDA’s database.
- Heart Attack: 35.86%
- Congestive Heart Failure: 27.86%
- Stroke: 15.66%
- Coronary Artery Disease: 10.02%
A 2007 study by cardiologist Steven Nissen and statistician Kathy Wolski in the New England Journal of Medicine first exposed Avandia’s association with severe heart-related side effects. Other studies have linked the drug to liver failure and an increased risk of pregnancy.
Of the more than 93,000 adverse events reported to the FDA, the agency classifies over 87% as “serious.”
| FDA Adverse Event Reports for Avandia | |
|---|---|
| Total cases reported | 93,820 |
| Serious cases (including deaths) | 81,975 |
| Deaths | 13,691 |
Disclaimer: Reports submitted to the FDA do not necessarily indicate that the drug caused an adverse event. It is important to consult with a health care professional before changing your medication.
After experts linked Avandia to heart-related side effects, its popularity plummeted. Due to these risks, the FDA temporarily imposed a restricted access program called Risk Evaluation and Mitigation Strategy (REMS) in 2011, limiting doctors’ ability to prescribe Avandia.
Other side effects of Avandia include bone fractures, liver toxicity, edema, weight gain, vision problems, and unexpected pregnancy risks.
“In my clinical practice, the most worrisome adverse effects of Avandia are congestive heart failure, myocardial infarction, and liver problems,” Dr. Michael McKinney, primary physician of the medical weight loss center Hea
- Abdominal pain
- Bone fracture
- Dark urine
- Discomfort in the chest that feels like pressure, squeezing, fullness or pain
- Menstrual cycle changes
- Pain or discomfort in the arms, back, neck, jaw or stomach
- Persistent nausea or vomiting
- Shortness of breath
- Swelling or weight gain
- Vision problems
- Yellowing of the eyes or skin
Less common adverse effects may include hypertension, upper respiratory tract infection, headache, back pain, high or low blood sugar, fatigue, sinusitis and diarrhea.
Manufacturer GlaxoSmithKline allocated billions of dollars to resolve thousands of Avandia lawsuits, which alleged that the company concealed the risks of heart attack and cardiac death from the public.
Avandia remains on the market in the United States.
Heart Health Concerns Lead To FDA Restrictions
FDA warnings and restrictions on Avandia have fueled the medication’s plummeting popularity. In 2007, the FDA required a black box warning about its potential to cause or worsen congestive heart failure.

Avandia can cause or worsen congestive heart failure in some patients.
Source: Avandia Prescribing Information
The FDA’s REMS program restricted the amount of Avandia prescriptions. In addition, negative media reports and increased medical skepticism drastically reduced Avandia’s use in treating Type 2 diabetes.
“The basic plot of the rosiglitazone story quickly became obvious to the advisory committee: A new ‘wonder drug,’ approved prematurely and for the wrong reasons by a weakened and underfunded government agency subjected to pressure from industry, had caused undue harm to patients,” Dr. Clifford J. Rosen, an endocrinologist and senior staff scientist at the Maine Center for Osteoporosis, wrote in
The New England Journal of Medicine. “The rosiglitazone story thus carries lessons for scientists, practitioners and regulators alike.”
In 2013, the FDA determined that follow-up data for rosiglitazone didn’t show it increased the risk of heart attack more than the standard Type 2 diabetes drugs metformin and sulfonylurea. The FDA discontinued the Avandia REMS in 2015 after manufacturers updated the label warnings about heart failure and provided educational training to doctors about its risks. Health professionals must monitor patients for rapid weight gain, breathing issues and swelling.
“I equip my clients with knowledge about the signs of heart failure, such as difficulty in breathing and swelling in their lower extremities, while stressing the importance of seeking immediate medical attention should any of these symptoms occur,” McKinney said.
The FDA has never banned or recalled Avandia, although the European Medicines Agency (EMA), its counterpart in the European Union, banned the drug in 2010 due to cardiovascular risks.
Did Avandia Cause Heart Attacks?
While there is still controversy about whether Avandia caused heart attacks, Nissen and Wolski’s 2007 study linked Avandia to a 43% increased risk of heart attacks. The study also found a 64% increased risk of death from cardiovascular causes among Avandia users.
If you are taking Avandia, watch out for the signs of a heart attack.
- Chest pain or pressure
- Feeling lightheaded
- Pain in the arms, legs, back, neck, jaw or stomach
- Shortness of breath
- Sudden fatigue or weakness
- Vomiting or nausea
Bone Fractures
Avandia showed an increased incidence of bone fractures in clinical trials, according to the drug’s label.
Long-term trials found an increased bone fracture risk in patients taking Avandia, especially women. Unlike typical osteoporosis sites, which are in the spine, hips and wrist, fractures caused by Avandia often occur in the upper arm, hand and foot.
This fracture risk persisted beyond the first year of treatment. Patients should monitor and maintain bone health while on Avandia.
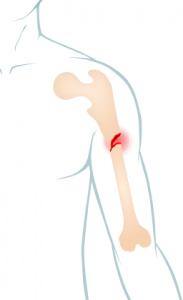
Long-term trials showed an increased rate of bone fractures in more than 200 patients, particularly women, who took Avandia.
Source: Avandia Prescribing Information
In a study published in the Archives of Internal Medicine, researchers found that patients with diabetes on Avandia had more than double the odds of fractures compared to patients who did not use this drug. Those who took Avandia for over a year had an even higher risk of bone fracture, especially in the hip and wrist. The study compared the records of 1,020 patients with diabetes and fractures against a control group of people with diabetes who did not have fractures.
“This analysis provides further evidence of a possible association between long-term use of thiazolidinediones and fractures, particularly of the hip and wrist, in patients with diabetes mellitus,” the authors wrote.
Liver Toxicity
Avandia’s label warns of possible liver toxicity, saying that doctors should not prescribe it to people with a history of liver problems. It also instructs doctors to check patients’ livers before and while on the drug.
A study in the journal LiverTox concluded that while Avandia has links to rare instances of liver injury, it has a much lower rate of liver enzyme elevation and liver injury than troglitazone, an antidiabetic and anti-inflammatory drug in the same class. Most liver issues resolved after stopping the medication, though there have been reports of fatal cases, according to the study.
Another case series in Pharmacoepidemiology & Drug Safety suggested that rosiglitazone and pioglitazone (Actos) may cause liver problems, citing 21 cases of drug-associated liver failure reported to the FDA between 1997 and 2006.
“This is the largest case series of liver failure associated with rosiglitazone or pioglitazone reported to date, strengthening the evidence that these drugs can cause severe hepatotoxicity [liver damage],” the authors wrote.
Doctors should be cautious about continuing treatment in patients with mild liver enzyme elevations and monitor them closely.
What To Do if You’re Experiencing Side Effects
If you suspect you are experiencing side effects from Avandia, it’s important to act promptly and responsibly. This includes speaking to your doctor or seeking emergency care if needed.
- 1. Contact Your Doctor — Do Not Discontinue on Your Own
- Do not stop Avandia without talking to your health care provider. Stopping abruptly may affect your blood sugar control. Describe your symptoms clearly so your doctor can assess the urgency and determine the next steps.
- 2. Get Cardiac Screening
- If you have any symptoms like chest pain, shortness of breath, swelling or irregular heartbeat, your doctor may recommend tests such as:
- • Electrocardiogram (ECG) to check heart rhythm and possible damage
- • Exercise stress test to evaluate heart function under exertion
- • Lipid panel to monitor cholesterol and related cardiovascular risks
- 3. Discuss Safer Alternatives for Type 2 Diabetes
- There are many other medications approved for Type 2 diabetes that may have a safer profile for you. Ask your doctor if switching to a different diabetes medication might benefit you.
- 4. Monitor for Signs of Fluid Retention and Chest Pain
- • Keep track of daily weight, any new swelling (especially in the feet, ankles or legs), trouble breathing or chest pain.
- • Report any rapid increase in weight, swelling or breathing difficulties immediately, as these may signal heart failure or fluid overload.
You may also consider reporting serious side effects to the FDA’s MedWatch program. This database helps track adverse events related to drugs and medical devices.
How Avandia Heart Risks Led To Lawsuits
After studies linked Avandia to an increased risk of heart attack and death, people filed thousands of lawsuits against GSK for concealing information that Avandia could cause heart attacks and strokes.
GSK paid $3 billion to the Justice Department over allegations that it unlawfully marketed several of its drugs, including Avandia. GSK also reportedly settled over 10,000 claims for $700+ million in 2011.
Learn more about Avandia lawsuits over heart risks.
Avandia Alternatives
Because there are many Avandia alternatives, the right treatment for you depends on your heart health, the side effects of each alternative, the cost and insurance coverage, the type of diabetes you have and the effectiveness of each drug.
- Actos (pioglitazone)
- Amaryl (glimepiride)
- DiaBeta, Glynase (glyburide)
- Farxiga (dapagliflozin)
- Glucotrol XL (glipizide)
- Glumetza, Glucophage (metformin)
- Insulin
- Invokana (canagliflozin)
- Januvia (sitagliptin)
- Jardiance (empagliflozin)
- Onglyza (saxagliptin)
- Ozempic (semaglutide)
- Rybelsus (semaglutide)
- Tradjenta (linagliptin)
- Victoza (liraglutide)
“I consider alternatives such as metformin, DPP-4 inhibitors, like sitagliptin, or GLP-1 receptor agonists, such as liraglutide, for patients at higher risk of the severe side effects of Avandia,” McKinney told Drugwatch. “The choice to go for other medication depends on the patient’s total profile, including their cardiovascular risks, kidney functionality and personal choices.”
“Metformin is always my first option because it has an improved safety record compared with other oral agents, and clinical outcome trials show that it reduces macrovascular complications in diabetics,” McKinney said.
A straightforward conversation with your doctor can help them determine your best alternative.
Calling this number connects you with a Drugwatch.com representative. We will direct you to one of our trusted legal partners for a free case review.
Drugwatch.com's trusted legal partners support the organization's mission to keep people safe from dangerous drugs and medical devices. For more information, visit our partners page.