Essure Birth Control: Side Effects, FDA Actions & Lawsuit Information
Essure, a permanent birth control device, was linked to severe side effects like bleeding, pain and organ perforation. Patient advocacy prompted an FDA response, and the manufacturer pulled it from the market in 2018. The device prompted thousands of lawsuits, which were settled for $1.6 billion.
Our content is developed and backed by respected legal, medical and scientific experts. More than 30 contributors, including product liability attorneys and board-certified physicians, have reviewed our website to ensure it’s medically sound and legally accurate.
legal help when you need it most.
Drugwatch has provided people injured by harmful drugs and devices with reliable answers and experienced legal help since 2009. Brought to you by The Wilson Firm LLP, we've pursued justice for more than 20,000 families and secured $324 million in settlements and verdicts against negligent manufacturers.
More than 30 contributors, including mass tort attorneys and board-certified doctors, have reviewed our website and added their unique perspectives to ensure you get the most updated and highest quality information.
Drugwatch.com is AACI-certified as a trusted medical content website and is produced by lawyers, a patient advocate and award-winning journalists whose affiliations include the American Bar Association and the American Medical Writers Association.
About Drugwatch.com
- 15+ Years of Advocacy
- $324 Million Recovered for Clients
- 20,000 Families Helped
- A+ BBB Rating
- 4.9 Stars from Google Reviews
Testimonials
I found Drugwatch to be very helpful with finding the right lawyers. We had the opportunity to share our story as well, so that more people can be aware of NEC. We are forever grateful for them.
- Legally reviewed by Jeffrey Johnson, J.D.
- Last update: December 2, 2025
- Est. Read Time: 9 min read
What Is Essure?
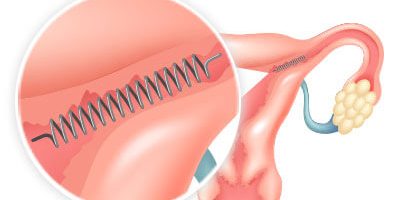
Essure is a permanent, non-surgical birth control device released by Conceptus (later purchased by Bayer). It looks like a spring and includes a stainless steel inner coil, a nickel-titanium expanding outer coil and a type of plastic fiber called polyethelene terephthalate (PET).
This now-discontinued birth control device was placed in the fallopian tubes during a quick, non-surgical office procedure. Over time, the coils would cause scar tissue to develop, blocking the tubes and preventing sperm from reaching and fertilizing eggs.
Approved by the U.S. Food and Drug Administration (FDA) in 2002, Essure was marketed as a less invasive alternative to tubal ligation. However, after it went into use, the FDA raised concerns about its safety and effectiveness.
Essure Side Effects and Complications
Following the FDA’s approval of Essure birth control, it was associated with a variety of side effects and complications. While issues like cramping and bleeding were supposed to be temporary, they sometimes lasted for years.
These situations and cases of device migration, fracture or breakage necessitated removal surgery.
Common Essure Short-Term Side Effects
Short-term side effects of Essure implantation included vaginal bleeding, abdominal pain, nausea and vomiting. It’s normal for mild-to-moderate side effects to accompany medical procedures, and the implantation of the Essure device was no exception.
- Abdominal pain
- Cramping
- Dizziness
- Nausea
- Risk of pregnancy in the first months after the procedure
- Vaginal bleeding
- Vomiting
Since it took time for scar tissue to build up around the device, there was a risk of pregnancy in the first few months following placement.
There were also risks specific to the implantation procedure. For example, a piece could break off the device during placement, necessitating the removal of the broken pieces.
Essure Confirmation Test Side Effects
The Essure Confirmation Test verified that the device was inserted correctly and would be effective at preventing pregnancy. The side effects of the test were similar to those reported during the implantation procedure.
- Cramping
- Dizziness
- Fainting
- Infection (rare)
- Nausea and vomiting
- Pain
- Spotting (rare)
To complete the Essure Confirmation Test, a health care provider performed a transvaginal ultrasound (TVU) or a vaginal X-ray exam. This test was completed three months after insertion.
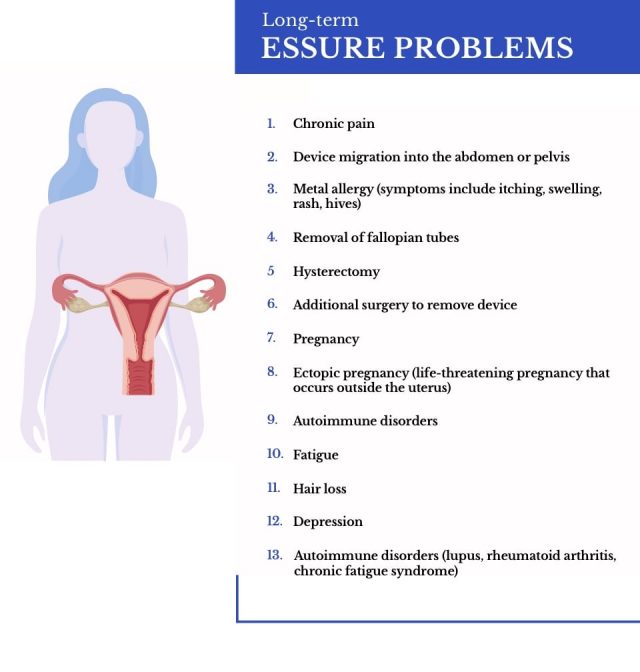
Long-Term Risks Reported by Essure Patients
Long-term risks of Essure birth control included severe organ perforation after the device migrated or fractured. These complications led to increased warnings.
- Abnormal Bleeding: While bleeding in the immediate aftermath of implantation was expected and generally mild, many women experienced irregular, heavy menstrual bleeding and suffered bleeding associated with severe abdominal pain.
- Chronic Pelvic Pain: One of the most common long-term symptoms women experienced was persistent and severe pelvic pain.
- Device fragmentation, fracture or breakage: Some Essure users had their devices fragment, fracture or break. This could lead to organ perforation or failure to prevent pregnancy.
- Device Migration: Many patients experienced a gradual migration of the Essure coils. This sometimes led to the coils puncturing nearby organs like the bladder, bowels or uterus.
From 2002 through 2024, more than 6,000 reports to the FDA’s Manufacturer and User Facility Device Experience (MAUDE) database mentioned device migration. Over 1,100 women reported that the device did not meet their expectations due to implant failure or unexpected pregnancy.
Essure Side Effects After 10 Years
Essure patients continued to report additional problems over time, raising concerns about the device’s safety. Many long-term side effects, such as pain and nickel sensitivities, persisted for years.
One of the key components of the Essure coils is nickel-titanium. Some patients reported sensitivity to this component, leading to issues like rashes.
“A lot of women had severe allergic reactions to the device — about 20% of the population is allergic to nickel and not aware of it,” Holly Ennis, an Essure lawyer and partner at Ennis & Ennis, told Drugwatch.
Due to sensitivity or device migration, sometimes patients had their Essure removed. Removal was often challenging, with reports of device fragmentation and retained coil pieces in the body. These remaining fragments could cause pain, allergic reactions or perforation.
FDA Warnings and Actions on Essure
The FDA has taken many actions concerning Essure, such as adding black box warnings, requiring additional safety studies and restricting its sales. However, because Essure’s most severe issues only emerged after years of use, the FDA took over a decade to take action.
In 2016, the FDA required a black box warning – the agency’s most serious safety alert – on Essure packaging. The warning informed patients of the risk for adverse events such as perforation of the uterus or fallopian tubes, persistent pain and suspected hypersensitivity.
The FDA also required Bayer to conduct a new study on the birth control risks of the Essure device compared to tubal ligation.
In 2017, the FDA announced an active evaluation of reports concerning Essure device removal.
In April 2018, the FDA restricted the sale of Essure devices to providers who agreed to review a checklist of the risks with patients before implantation. This checklist would ensure that patients were fully informed of the risks and possible side effects of Essure.
In July of that year, Bayer informed the FDA that Essure birth control would no longer be sold or distributed after December 31, 2018. All unused Essure devices should have been returned to Bayer as of December 31, 2019.
From January 2, 2022, to December 31, 2024, the FDA received over 73,000 adverse event reports from patients and doctors detailing complications from Essure.
Bayer Discontinued Essure
Bayer voluntarily stopped the sale and distribution of Essure at the end of 2018, citing declining sales. In the years leading up to the discontinuation, pressure from regulators and patients drew attention to the risks of Essure. This may have contributed to its sales decline.
The widespread harms and negative attention associated with Essure were underscored by thousands of lawsuits. Bayer went on to pay $1.6 billion to settle nearly 90% of the approximately 39,000 claims in the U.S.
By December 31, 2019, all unused devices should have been returned to Bayer. Today, the device is no longer distributed or used in the United States.
Essure Removal Procedures
Essure devices were intended to be a permanent form of birth control, making removal complicated. It can only be removed surgically.
Removing the fallopian tubes was one surgical option. However, because part of the device overlaps with the uterine muscle, the safest way to remove Essure birth control and minimize the risk of device fracture was through a complete hysterectomy (removal of the uterus).
According to the FDA, 10% of the over 10,000 device removal reports the agency received between 2016 and 2018 reported complications from Essure removal involving:
- Coil migration
- Device breakage
- Device fragments remaining in patients
- Perforation of the uterus or fallopian tube
- Severe bleeding during or after surgery
Notably, less than 50% of the reports claimed that all the patient’s symptoms from the Essure device were resolved after device removal.
| Outcome of Device Removal | |
|---|---|
| Patient Symptoms Resolved After Device Removal | 43% |
| Patient Symptoms Partially Resolved After Device Removal | 45% |
| Patient Symptoms Not Resolved or Improved | 12% |
Should You Remove Your Essure?
If you have Essure birth control and are experiencing side effects, such as debilitating pain, or you don’t feel you can rely on the implant to prevent pregnancy, talk to your doctor about removing it.
According to the FDA, most patients remove their Essure due to pain, genital hemorrhage, perforation, allergies to metal, breakage and dislocation, migration or expulsion. If you have any of these complications, it might be wise to remove the device.
Current Essure users who are not experiencing adverse effects do not need to have the device removed. The FDA assures that “Women who have been using Essure successfully to prevent pregnancy can and should continue to do so.”
Legal Controversies and Essure Lawsuits
The declining sales that led to Essure’s market removal were likely caused by the voices of thousands of injured women who sought justice through the courts.
Patients filed lawsuits alleging that Essure carried severe, undisclosed risks and that the product was defective in its design. Many of these patients experienced severe pain for years, even after the device was removed.
Why Women Filed Lawsuits
The Essure lawsuits consisted of allegations of defective design and failure to warn. These claims formed the legal basis for holding the manufacturer, Bayer, accountable.
- Defective Design
- Plaintiffs alleged that the Essure device was inherently unsafe and prone to breaking, corroding and migrating from its intended location. This could lead to severe internal injuries, such as the coils puncturing the uterus or other nearby organs. The claim argued that the product’s design was fundamentally dangerous, rather than just due to a manufacturing error.
- Failure to Warn
- Lawsuits claimed that the manufacturer failed to inform patients and doctors about the device's serious risks and complications. Plaintiffs argued that if the public had been aware of the potential for chronic pain, bleeding and device migration, doctors and patients would have chosen different birth control options.
Bayer attempted to dismiss many of these cases using the defense of federal preemption, basically claiming that FDA approval protected them from state-level lawsuits.
However, many plaintiffs successfully countered that this protection was forfeited because the company allegedly misrepresented the device’s safety to the FDA and failed to report all adverse events as required.
Litigation Status
There were nearly 40,000 total lawsuits filed involving Essure devices. Many of the claims were consolidated in different states, primarily in California and Pennsylvania.
In August 2020, Bayer agreed to a $1.6 billion settlement to resolve most of the Essure lawsuits. According to Reuters, the settlement will cover nearly 90% of the claims in the U.S.
As part of the settlement, Bayer admits no wrongdoing or liability.
Patient Advocacy and Real Stories
Women like Angie Firmalino and Theresa Sawyer helped raise awareness for thousands of patients affected by adverse side effects allegedly caused by Essure.
Angie Firmalino founded the Facebook group “Essure Problems,” which became a focal point for women to share experiences and support. Firmalino was implanted with Essure and suffered years of pain and bleeding before founding the group, which has accumulated over 43,000 members.
Firmalino was also one subject of the 2018 Netflix documentary “The Bleeding Edge.” The film focused on multiple medical devices, including Essure. One week before the documentary’s premiere, Bayer announced the end of Essure sales and distribution in the U.S.
Theresa Sawyer was another leader in the efforts to reveal the dangers of Essure. Her experiences as one of the first women to use Essure – participating in a trial before it was approved in 2002 – and one of the first women to suffer complications, helped validate other people’s experiences as the community came together.
FDA Commissioner Scott Gotlieb met with Firmalino and other members of the “Essure Problems” Facebook group in February 2018. Two months later, stricter guidelines were implemented for using Essure. Five months after that, Bayer announced the end of Essure use in the United States.
Amanda Chavarria had Essure implanted when she was 22. After the procedure, she began having pelvic pain and irregular menstrual cycles. Then, back pain, fatigue, rashes, swelling and migraines started. She also became depressed and experienced an ectopic pregnancy.
Chavarria reported that she planned to get her Essure removed after fearing that she’d be struggling with health issues the rest of her life.
Essure Alternatives
While Essure is no longer on the market, there are other IUDs for those seeking long-term or permanent birth control. Some IUDs, including Mirena, Kyleena, Liletta and Skyla, are hormonal. Others, like Paragard, are copper.
Like Essure, a medical practitioner inserts these devices through the vagina. Most IUDs need to be replaced every five to 10 years, depending on the brand. IUDs also share many of the same risks as Essure, including penetration of the uterine lining.
Tubal ligation, which blocks, clips or removes your fallopian tubes, is another form of lasting birth control. However, since this is a more invasive procedure, you and your doctor should discuss any potential risks or relevant health factors related to this surgery.
Key Takeaways for Patients
Essure serves as a vital case study in medical device safety and patient advocacy. For those who have the device, it’s important to know that issues can arise years later. It is crucial to consult with your doctor if any new symptoms appear, including pain, bleeding or other side effects.
The device’s controversy exposed significant issues. These included the severe risks that were not fully disclosed during its initial approval and the reactive, rather than preventative, response from Bayer and the FDA.
Ultimately, the collective power of thousands of people who filed lawsuits resulted in a $1.6 billion settlement from Bayer, providing a measure of justice for those who were harmed.
For more information, visit our Essure lawsuits page.
Calling this number connects you with a Drugwatch.com representative. We will direct you to one of our trusted legal partners for a free case review.
Drugwatch.com's trusted legal partners support the organization's mission to keep people safe from dangerous drugs and medical devices. For more information, visit our partners page.