Promethazine
Promethazine (promethazine hydrochloride) is an antihistamine used to treat allergic reactions like sneezing, runny nose, watery eyes and skin rashes. Promethazine may also be prescribed for nausea, vomiting or presurgical sedation.
What Is Promethazine?
Promethazine is an older antihistamine. People usually take it as promethazine hydrochloride, a more stable salt form of the drug. Promethazine is not a narcotic, but it is a controlled substance. As a result, the U.S. Drug Enforcement Administration identifies it as having a higher-than-normal risk of abuse.
Promethazine is only available in the U.S. as a prescription. It is manufactured into tablets, capsules and liquid medication.
People who take promethazine, which also sells under the brand names Phenergan and Promacot, can mix it with other prescription and over-the-counter drugs that treat coughs and colds. However, taking promethazine with cough and cold medications or medicines that contain codeine can increase its sedative or respiratory depressive effects and abuse potential.
What Does Promethazine Treat?
Promethazine treats allergic reactions and mild to moderate allergy symptoms, including hay fever and allergic conjunctivitis. It reduces the effects of histamine, a product of the immune system. People may take promethazine after allergy symptoms begin or to prevent symptoms from occurring.
Medical providers sometimes prescribe promethazine to manage nausea, vomiting and related problems such as motion sickness, dizziness and vertigo. It can also induce sleep, control pain or relieve anxiety before a medical procedure.
- Assist with surgery in special situations.
- Calm patients in medical settings, such as before surgery or during labor.
- Control nausea and vomiting after certain surgeries.
- Manage pain after surgery.
- Prevent and treat motion sickness.
- Treat allergic conjunctivitis (red, watery eyes).
- Treat allergic reactions to blood or plasma products.
- Treat allergic rhinitis (runny nose and watery eyes).
- Treat allergic skin reactions (rash, hives).
People also use promethazine cough syrup to treat cold symptoms or respiratory infections. However, the syrup form of promethazine typically contains codeine, a strong opiate painkiller. Therefore, it is also only available as a prescription.
Scientists are exploring new uses for promethazine, including as a potential future treatment for strokes and cancer.
How Does Promethazine Work?
Promethazine blocks the histamine your body makes during allergic reactions. Histamine causes sneezing, a runny nose and other symptoms associated with allergies. Blocking it relieves these symptoms.
Promethazine also halts the neurotransmitter dopamine to manage nausea and other related issues.
How Is Promethazine Administered?
You can take promethazine as a tablet or liquid. It is administered through the rectum as a suppository or into the muscle via injection.
People who prefer liquid promethazine should not use a household spoon to measure their dosage. Instead, use a measuring spoon or cup to ensure the correct dose.
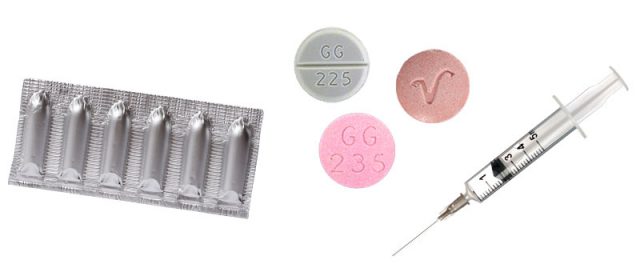
To use in a suppository, dip the tip of the suppository tablet in water and insert it approximately one inch into your rectum.
Injecting promethazine can cause severe chemical irritation and tissue damage. Therefore, promethazine injections should only be given via deep intramuscular injection.
Promethazine Dosages
Promethazine may be prescribed in various doses depending on its treating condition. Always follow your doctor’s directions when taking this drug.
Promethazine typically kicks in after about 20 minutes, regardless of the dose. However, rapid absorption causes the drowsiness associated with the drug. Promethazine works for up to six hours.
- Allergic Conditions: 25 mg is the average dose for allergies in adults.
- Before Surgery: Adults take 50 mg before surgical or obstetric procedures.
- Nausea and Vomiting: Adult patients usually take 25 mg every 4-6 hours.
- Sedation: Hospitalized adults may take 25 to 50 mg as an injection to achieve sedation.
Children typically receive 0.5 mg of promethazine per pound of body weight.
It is possible to overdose on promethazine. Overdosing produces alarming symptoms, such as seizures and severely low blood pressure. Promethazine overdose is rarely fatal but taking too much is risky.
Promethazine Side Effects
The most prevalent side effects of promethazine are drowsiness, restlessness and dizziness. Fortunately, most side effects go away within a few weeks after taking the drug.
- Atypically happy mood
- Blurred vision or double vision
- Difficulty falling or staying asleep
- Dizziness
- Drowsiness
- Dry mouth
- Hyperactivity
- Itching
- Nausea
- Nervousness
- Nightmares
- Stuffy nose
- Vomiting
Promethazine is also associated with rare long-term side effects, including heart arrhythmias, low blood pressure, liver damage, bone marrow suppression and trouble regulating body temperature. It can also cause skin hyperpigmentation after long-term use, especially on body parts that are exposed to the sun.
- Abnormal sweating
- Abnormal neck or tongue position
- Confusion
- Extreme anxiety
- Faintness
- Fever
- Hallucinations
- Hives
- Rapid or irregular heartbeat
- Rash
- Seizures
- Slowed or stopped breathing
- Stiff muscles
- Swelling of the face, eyes, throat, lips, tongue, hands, feet or ankles
- Tremors
- Uncontrolled eye movements
- Unusual bleeding or bruising
- Wheezing
- Yellowed eyes or skin
These serious side effects are rare. Tell your doctor if you experience any of them.
Promethazine's Black Box Warnings
Injectable products that contain promethazine hydrochloride have two black box warnings on their label. These labels warn of two instances where using these products poses a significant risk of injury or death.
The first addresses possible respiratory depression, or slowed breathing, in children under age 2. The second warns of possible severe tissue injury, including gangrene.
Warnings for Pediatric Use
Promethazine is linked to serious side effects in children, such as respiratory depression, oversedation and seizures. These effects can be fatal.
The U.S. Food and Drug Administration began requiring manufacturers to include a black box warning detailing these side effects in 2004. They advise against promethazine for children younger than 2. The FDA also recommends caution when administering the drug to older children.
Promethazine’s Risk of Tissue Injury
Promethazine hydrochloride injections may also cause severe tissue injury, including gangrene and necrosis. As of 2009, promethazine carries a second black box warning describing this risk.
The FDA now advises health care providers to administer promethazine injections intramuscularly rather than into the veins or arteries. The Institute for Safe Medication Practices also recommends that hospitals stop injecting promethazine.
Promethazine & Drug Interactions
Promethazine may interact with other drugs you are taking. It can decrease their effectiveness or increase unwanted side effects. Discuss promethazine’s interactions with your doctor before you take it.
- Acetylcholinesterase Inhibitor Medications: These drugs treat dementia in people with Alzheimer's disease.
- Alcohol: Even a small amount of alcohol can amplify the sedative effects of promethazine.
- Anticholinergic Drugs: These medications treat conditions such as dizziness, respiratory disorders, gastrointestinal disorders and insomnia.
- Antidepressants: CYP2D6 inhibitor medications such as Prozac (fluoxetine) and CYP2B6 inhibitor medications such as Paxil (paroxetine) treat mental health conditions like depression and anxiety.
- Epinephrine: A natural hormone, also known as adrenaline, epinephrine sometimes treats severe allergic reactions.
- Monoamine Oxidase Inhibitors (MAOIs): These medications are prescribed for depression, panic disorder and social anxiety.
- Pramlintide (Symlin): Pramlintide helps control blood sugar in people with diabetes.
- Other Medications That Cause Drowsiness. Anxiety and antipsychotic medications, muscle relaxants and narcotic pain relievers, most antidepressants, and some antihistamines can worsen the drowsiness caused by promethazine.
Give your doctor a complete list of all your medications before adding anything new. Don’t take substances or over-the-counter medications that could interact with the promethazine.
Calling this number connects you with a Drugwatch.com representative. We will direct you to one of our trusted legal partners for a free case review.
Drugwatch.com's trusted legal partners support the organization's mission to keep people safe from dangerous drugs and medical devices. For more information, visit our partners page.


