Zantac
Zantac (ranitidine) is used to treat or relieve heartburn, acid indigestion, GERD and gastric ulcers. It belongs to a class of drugs called H2 blockers. The FDA issued a recall after Zantac made with ranitidine was found to contain the carcinogen NDMA. A reformulated Zantac, Zantac 360, is currently available with famotidine replacing ranitidine.
- Medically reviewed by Samantha Spencer, Pharm.D., BCPS
- Last update: March 7, 2025
Is Zantac Still Available?
The antacid Zantac — known as ranitidine in generic form — was available over the counter or as a prescription in the United States until April 2020. You can no longer buy ranitidine products in the U.S.
After the market withdrawal of ranitidine products, Sanofi reformulated an over-the-counter version of Zantac 360 made with famotidine. The new Zantac 360 formula with famotidine is available in regular strength and maximum strength in the U.S.
Unlike the old Zantac formula made with ranitidine, the new Zantac 360 doesn’t come in prescription strength.
Consumers can safely buy and use Zantac 360 with famotidine wherever OTC medications are sold. The FDA hasn’t found any NDMA contamination in famotidine and has deemed it a safe alternative to the old ranitidine formula.
This page presents information about Zantac made with ranitidine.
Why Was Zantac (Ranitidine) Recalled?
Zantac made with ranitidine was recalled because the FDA found traces of N-Nitrosodimethylamine (NDMA) — a chemical impurity that can cause cancer — in some batches of the drug.
Beginning in September 2019, manufacturers and pharmacies such as CVS, Walgreens and Walmart pulled the drug off store shelves. In April 2020, the FDA requested all ranitidine products be removed from the market.
The International Agency for Research on Cancer has said NDMA is “probably carcinogenic to humans.” It’s classified as a group 2A carcinogen because there is sufficient evidence that it could cause cancer in humans. In studies, NDMA was also linked to cancers of the breast, colon, esophagus, kidney, liver, skin, ovaries, prostate and stomach.
After the Zantac recalls and market withdrawal, lawsuits were filed against the drug’s manufacturers, including Sanofi. Zantac lawsuits claimed the drug caused plaintiffs to develop bladder cancer. In December 2022, a federal judge dismissed the lawsuits, citing lack of evidence. Plaintiffs planned to appeal.
Zantac isn’t the only drug that has been recalled for NDMA contamination. The FDA announced recalls of valsartan, losartan and metformin because some batches tested positive for the toxic chemical. In August 2022, the FDA announced it would allow temporary distribution of sitagliptin contaminated with Nitroso-STG-19, a chemical related to NDMA.

How Does Zantac (Ranitidine) Work?
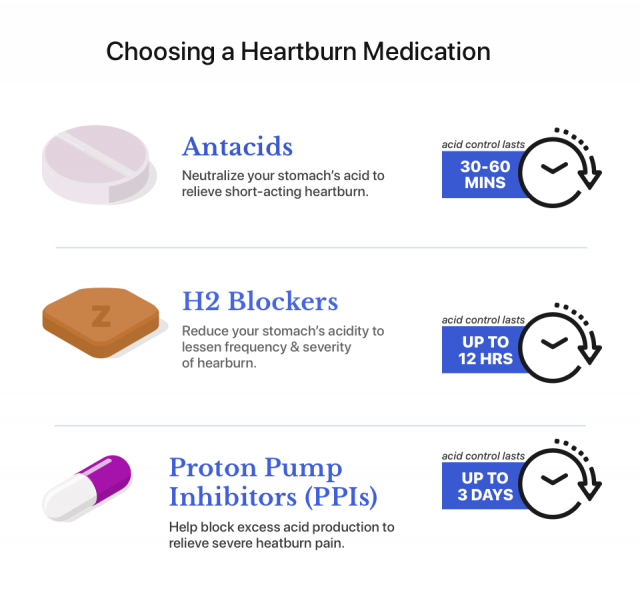
Zantac (ranitidine) belongs to a class of acid-blocking drugs called H2 blockers. The active ingredient, ranitidine, works by blocking a chemical called histamine. This reduces the amount of acid in the stomach.
The drug starts to work in as little as 30 minutes. It can control acid production for up to 12 hours and reduce the frequency and severity of heartburn.
Zantac made with ranitidine was used to treat a variety of gastrointestinal disorders, including heartburn, GERD and gastric ulcers.
How To Take Zantac
Over-the-counter and prescription versions of Zantac made with ranitidine have different usage instructions. Depending on the reason for its use, the dosages will vary.
Hospitals use the Zantac injection for patients with pathological hypersecretory conditions or intractable duodenal ulcers, or as an alternative to the oral medication.
OTC Directions
Over-the-counter Zantac treats heartburn with acid indigestion and sour stomach caused by eating or drinking certain foods or beverages. Children under 12 should not use Zantac without first getting approval from a health care provider.
- Swallow one tablet with a glass of water for symptom relief.
- To prevent heartburn, swallow one tablet with a glass of water 30 to 60 minutes before eating or drinking.
- Do not take more than two tablets in 24 hours.
- Do not chew tablet.
Prescription Directions and Recommended Dosages
Prescription Zantac treats several acid-related conditions, from GERD to erosive esophagitis.
Children as young as 1 month old may take the drug, but researchers have not studied it in children younger than 1 month of age and cannot make dosing recommendations.
Providers should adjust the dosage for people with impaired kidney function.
| Indication | Dose/Instructions |
|---|---|
| Active Duodenal Ulcer | 150 mg twice daily or 300 mg once daily after the evening meal or at bedtime |
| Maintenance of Healing of Duodenal Ulcers | 150 mg at bedtime |
| Pathological Hypersecretory Conditions (such as Zollinger-Ellison syndrome) | 150 mg twice a day. Some patients may need to administer ranitidine 150 mg doses more frequently. Providers may increase the dosage up to 6 g a day. |
| Benign Gastric Ulcer | 150 mg twice a day |
| Maintenance of Healing of Gastric Ulcers | 150 mg at bedtime |
| GERD | 150 mg twice a day |
| Erosive Esophagitis | 150 mg four times a day |
| Maintenance of Healing of Erosive Esophagitis | 150 mg twice a day |
| Pediatric Treatment of Duodenal and Gastric Ulcers | 2 to 4 mg/kg twice daily to a maximum of 300 mg/day |
| Pediatric Maintenance of Healing of Duodenal and Gastric Ulcers | 2 to 4 mg/kg once daily to a maximum of 150 mg/day |
| Pediatric Treatment of GERD and Erosive Esophagitis | 5 to 10 mg/kg per day, usually given as two or three divided doses |
Side Effects of Zantac (Ranitidine)
Most Zantac side effects are typically mild and pass quickly. The most commonly reported adverse events include constipation, diarrhea and nausea or vomiting.
Rarely, some serious side effects such as skin rashes, liver failure and cardiac disorders may occur. There have been rare reports of agitation, confusion, depression and hallucinations in severely ill, elderly patients.
Zantac Warnings & Precautions
People with kidney disease, impaired renal function or liver problems should tell their doctors about their condition because ranitidine is excreted by the kidneys and metabolized by the liver, the drug’s label warns.
In addition, ranitidine may cause severe attacks in people with a history of acute porphyria and people with this disease shouldn’t take Zantac with ranitidine.
What Cancers Does Zantac Cause?
Zantac and other ranitidine products contaminated with high levels of NDMA, a probable human carcinogen, could cause many types of cancer, including bladder cancer, colon cancer and prostate cancer. It’s important to know that the research on this topic is still new and scientists haven’t found solid evidence that says Zantac causes any type of cancer. But they cannot rule out the drug’s cancer-causing potential either.
According to lawyers accepting Zantac cancer cases, many people who developed cancer after taking ranitidine didn’t have a family history of cancer or genetic markers for the disease. Their treating physicians stressed that the cancer was caused by something environmental. NDMA is an environmental contaminant.
Types of cancer caused by Zantac include:
- Bladder cancer and bladder removal
- Breast cancer
- Colon cancer
- Kidney cancer and kidney removal
- Liver cancer
- Prostate cancer
- Stomach cancer
Cancer isn’t a side effect listed on Zantac’s drug label, but some studies have suggested that high levels of NDMA in Zantac may cause cancer.
One such analysis published in JAMA in March 2021 by Dr. Lior Z. Braunstein and colleagues suggests ranitidine may form toxic levels of NDMA in the body when exposed to conditions in the human stomach.
Researchers found ranitidine was capable of producing NDMA levels far above the FDA’s acceptable limits.
Zantac Drug Interactions
Tell your health care provider about all medications, herbal supplements and vitamins you take before taking Zantac. Avoid drinking alcohol with the drug because it may increase the risk of stomach damage.
Because ranitidine affects stomach acid, it may increase or decrease the effectiveness of drugs that rely on stomach acid to be effective.
There are no interactions listed for Zantac OTC, but the prescription drug label includes several.
- Atazanavir
- Drug absorption may be impaired, use with caution.
- Delavirdine
- Drug absorption may be impaired, use with caution.
- Gefitinib
- Gefitinib exposure was reduced when used with ranitidine, use with caution.
- Glipizide
- Glipizide exposure increased after a single 150-mg dose of oral ranitidine in diabetic patients.
- Ketoconazole
- Oral ketoconazole exposure was reduced by up to 95% when used with ranitidine.
- Midazolam
- Oral midazolam increased when used with ranitidine and may cause excessive and prolonged sedation.
- Procainamide
- Higher doses of ranitidine (> 300 milligrams/day) may increase plasma levels of this drug and in rare cases may cause toxicity.
- Triazolam
- Oral triazolam increased when used with ranitidine and may cause excessive or prolonged sedation.
- Warfarin
- Ranitidine may affect warfarin’s effectiveness.
Zantac Alternatives
Ranitidine is no longer available in the U.S., but people who depended on the drug to control acid reflux and other acid-related disorders have a few Zantac alternatives.
Zantac 360 with famotidine is currently available. Medication alternatives include proton pump inhibitors and other H2 blockers. People who want to switch medications should speak to their medical provider.
Proton Pump Inhibitors
Proton pump inhibitors are powerful acid-reducing drugs that can control acid for up to 24 hours if taken every day.
- Nexium (esomeprazole)
- Prevacid (lansoprazole)
- Prilosec (omeprazole)
H2 Blockers
The FDA has tested other H2 blockers and did not find NDMA contamination. FDA-recommended H2 blockers include Pepcid (famotidine) and Tagamet (cimetidine). In addition, Sanofi has released a new over-the-counter Zantac 360 formula made with famotidine, the same active ingredient in Pepcid.
These drugs work similarly to Zantac with ranitidine, but each has slightly different side effects. Pepcid has fewer common side effects than Tagamet and is more tolerable.
Pepcid’s most common side effect is headache, while Tagamet has a longer list. These include rashes, diarrhea, dizziness, headache and gynecomastia — a disorder that causes men to grow breasts.
| Drug | How It Works | How Long it Takes to Work | How Long It Lasts |
|---|---|---|---|
| Zantac (H2 Blockers) | Reduces stomach acid production, can provide immediate relief | 30 minutes to an hour | Up to 12 hours |
| Pepcid (famotidine) (H2 Blockers) | Reduces stomach acid production, can provide immediate relief | 30 minutes to an hour | Up to 12 hours |
| Prilosec (Proton Pump Inhibitor PPI) | Blocks acid production, treats frequent heartburn and not intended for immediate relief | May take 1 to 4 days to work | Up to 24 hours when used daily |
In clinical trials, Zantac (ranitidine) and Pepcid (famotidine) perform similarly when it comes to reducing stomach acid.
However, in some of the oldest studies comparing the drugs, researchers found famotidine is 7.5 times more potent than ranitidine and may be slightly more effective at reducing stomach acid.
Calling this number connects you with a Drugwatch.com representative. We will direct you to one of our trusted legal partners for a free case review.
Drugwatch.com's trusted legal partners support the organization's mission to keep people safe from dangerous drugs and medical devices. For more information, visit our partners page.