Varubi
Varubi (rolapitant) prevents nausea and vomiting in cancer patients receiving chemotherapy. The U.S. Food and Drug Administration in January 2018 issued a warning about the potential for the injectable form of Varubi to cause serious allergic reactions. Lawyers are filing class-action lawsuits on behalf of shareholders. They may also investigate individual injury claims.
The medication, which was formerly made by Boston-based Tesaro and is now marketed by TerSera Therapeutics, comes in oral tablets. The liquid for intravenous (IV) use has been discontinued in the United States. The active ingredient is called rolapitant.
The FDA approved Varubi tablets in 2015 and injections in 2017.
On January 16, 2018, the FDA warned that Varubi may cause allergic reactions minutes after being administered intravenously. Some patients required hospitalization.
Tesaro sent a warning letter to health care providers in January 2018. Doctors administered about 7,000 doses of the injection from October 2017 to January 2018, according to Tesaro.
As of 2022, Varubi lawyers are filing class action lawsuits for Tesaro shareholders. Tesaro knew the drug could cause allergic reactions and failed to disclose the risk, lawyers say.
Lawyers may also be investigating individual Varubi allergic reaction injury claims.
How Varubi Works
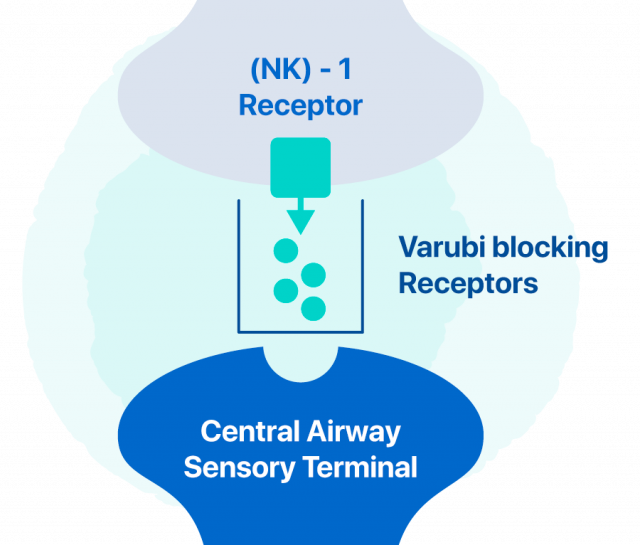
Varubi works by blocking receptors in the body that play a role in vomiting and nausea. These receptors are called neurokinin (NK)-1 receptors.
(NK)-1 receptors are located in the brain stem. Varubi crosses the blood-brain barrier and interferes with the receptors. Doctors use Varubi with other medications to prevent nausea and vomiting caused by chemotherapy.
Varubi belongs to a class of drugs called (NK)-1 receptor antagonists. Other drugs in the same class are Emend (aprepitant or fosaprepitant, manufactured by Merck), Cinvanti (aprepitant, manufactured by Heron Therapeutics) and Akynzeo (netupitant/palonosetron or fosnetupitant/palonosetron, manufactured by Helsinn Therapeutics).
Varubi Side Effects
The most serious Varubi side effects reported are anaphylaxis and anaphylactic shock with IV Varubi. These are types of allergic reactions.
These side effects can occur within minutes after receiving IV Varubi. Most people who developed these side effects had to go to the hospital.
Less serious Varubi side effects may vary depending on the type of chemotherapy a patient receives. These may occur with oral or IV Varubi. Some are immediate, while others may surface later.
- wheezing
- difficulty breathing
- swelling of the throat or face
- hives or flushed skin
- itching
- abdominal pain or cramping
- vomiting
- back or chest pain
- feeling faint
- neutropenia (low white blood cell count)
- hiccups
- abdominal pain
- decreased appetite
- dizziness
- dyspepsia (indigestion)
- urinary tract infection
- stomatitis (mouth sores)
- anemia (low red blood cell count)
Varubi FDA Warnings
On January 16, 2018, the FDA released a safety communication warning that Varubi infusions could lead to serious hypersensitivity reactions such as anaphylaxis and anaphylactic shock.
These reactions occurred during or soon after receiving IV Varubi, according to the FDA. Most reactions occurred within a few minutes of receiving the drug.
One of the ingredients in Varubi is soybean oil. People allergic to soybeans should tell their doctor before using Varubi.
Tesaro announced changes to the drug’s label to add information about hypersensitivity reactions on January 12, 2018.
Varubi Dosages, Recommendations and Costs
Patients receive Varubi with other types of anti-nausea drugs. They receive the first dose two hours before starting chemotherapy on the first day.
For tablets, the recommended dose is 180 mg.
The average cost of Varubi is about $560 for two tablets (90 mg per tablet).. The price may change depending on insurance plans.
Varubi stays in the body for seven days, longer than a cheaper drug in the same class. Emend (aprepitant), another (NK)-1 receptor antagonist, costs about $130 for one pill.
Varubi Drug Interactions
Varubi interacts with drugs that increase or decrease levels of an enzyme in the body called CYP2D6. Many antidepressants interact with this enzyme.
Patients taking thiorodazine or pimozide should not take Varubi. Taking these drugs together may lead to life-threatening abnormal heart rhythms.
Other drugs that may interact with Varubi include Prozac (fluoxetine), Paxil (paroxetine),rifampin, digoxin, tamoxifen and codeine.
Alternatives to Varubi
People who cannot take Varubi may try other drugs to prevent chemotherapy-induced nausea and vomiting.
For example, other drugs in the same class include Emend (aprepitant or fosaprepitant), Cinvanti (aprepitant and Akynzeo (netupitant/palonosetron or fosnetupitant/palonosetron). Other drugs that prevent nausea due to chemotherapy include Decadron (dexamethasone), Zofran or Zuplenz (ondansetron), Aloxi (palonosetron), Sustol or Sancuso (granisetron) and Reglan (metoclopramide).
Other options used to control nausea are drugs that mimic the effects of cannabis. These drugs are called synthetic cannabinoids. These include Marinol and Syndros (dronabinol) and Cesamet (nabilone).
These drugs have their own side effects. Patients should talk to their doctors before taking these medications.
Varubi Lawsuit and Class Action
Robert Bowers filed a class-action complaint in Massachusetts against Tesaro and some of its officers on January 17, 2018.
The complaint alleged Tesaro failed to disclose the risk of anaphylaxis and anaphylactic shock. This led to artificially inflated stock prices.
After the FDA released the side-effect warning, stock prices fell by about $4.07. As a result, “class members suffered significant loses and damages,” the complaint said.
Lawyers may also be accepting individual cases from patients who experienced anaphylaxis and anaphylactic shock after taking Varubi.
Calling this number connects you with a Drugwatch.com representative. We will direct you to one of our trusted legal partners for a free case review.
Drugwatch.com's trusted legal partners support the organization's mission to keep people safe from dangerous drugs and medical devices. For more information, visit our partners page.

