Viberzi Lawsuits
Patients who took Viberzi and suffered pancreatitis are filing lawsuits against its manufacturer, Allergan. The lawsuits claim Allergan failed to warn doctors that Viberzi increases the risk of pancreatitis in patients without gallbladders. Lawsuits also allege that there were signs of potential problems with the drug even before the FDA issued a warning in 2017.
Latest Status of Viberzi Lawsuits
There have been no major settlements or verdicts in Viberzi lawsuits. As of July 2024, all cases have been filed as individual lawsuits and have not been consolidated into a multidistrict litigation. Drugwatch’s partners are not currently accepting Viberzi lawsuits.
Viberzi lawsuits allege that Allergan failed to warn about the increased risk of potentially fatal pancreatitis in patients without a gallbladder who were prescribed Viberzi.
Symptoms of pancreatitis include abdominal pain, inflammation, pancreas damage, and complications affecting vital organs. Severe pancreatitis can be life-threatening, with instances culminating in fatalities.
Viberzi Patient Experience
When Erin Duncan was prescribed Viberzi in 2016, she hoped the medication would relieve her symptoms of irritable bowel syndrome. But after taking just one dose of the drug, the 46-year-old California woman landed in the hospital.
Lab tests and imaging showed that Duncan was suffering from acute pancreatitis, an inflammation of the pancreas. The condition is serious and sometimes deadly. It can lead to complications such as kidney failure, respiratory failure, diabetes and pancreatic cancer — and Duncan says Viberzi maker Allergan is entirely to blame.
In September 2018, Duncan filed an eight-count lawsuit against Allergan in U.S. District Court for the Central District of California.
In her suit, she alleges the drugmaker failed to “adequately test for and warn” that Viberzi created an increased risk of pancreatitis in patients like herself who don’t have gallbladders.
Though Duncan was discharged from the hospital after four days, she says she “remains at an increased risk” for recurrent attacks of pancreatitis and lives in fear she may one day develop pancreatic cancer because of the inflammation.
As of early October 2018, Allergan had not yet responded to the initial complaint filed by Duncan, which is among the first Viberzi suits filed over the drug’s side effects.
The company had 21 days to respond to the allegations, according to court filings.
Similar lawsuits could follow. According to the FDA, outpatient retail pharmacies in the U.S. dispensed about 64,000 Viberzi prescriptions from May 2015 through July 2016, though it’s unclear how many people developed pancreatitis while taking the drug.
Gallbladder Problems Spark FDA Warning
When the FDA approved Viberzi in 2015, the drug was hailed by its manufacturers as a “first step to providing physicians with a new, evidence-based, treatment option” for adults with irritable bowel syndrome with diarrhea (IBS-D).
But nearly two years later, in March 2017, the FDA issued a warning that the drug should not be used in patients who’ve had their gallbladders removed.
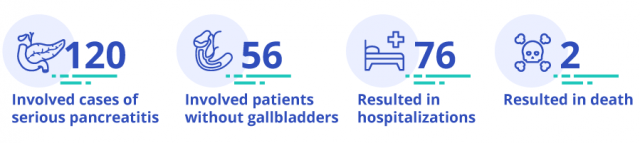
The regulatory agency said it had received 120 reports between May 2015 and February 2017 of patients who’d developed serious cases of pancreatitis while taking Viberzi. Seventy-six of those patients ended up in the hospital and two died. At least 56 of the ill patients — including the two who died — did not have their gallbladders.
The European Medicines Agency, the European Union’s equivalent to the FDA, issued similar warnings about the drug even earlier, in July 2016.
Reports to FDA Between May 2015 & February 2017
Suit Says Clinical Trials Raised Red Flags
Litigants, such as Duncan, allege that there were signs of potential problems with the drug even before the FDA warning — and contend that the drugmaker did not properly investigate them.
Duncan’s complaint notes that at least 40 patients participating in Vibrezi clinical trials stopped taking the drug because of abdominal pain. According to the lawsuit, half of those events occurred during the first 24 hours of therapy, but the “vast majority” of those patients were not tested to see if they were suffering from pancreatitis.
“Properly designed and executed clinical trials would have led the original May 2015 label to contraindicate use in patients without gallbladders, Because the FDA did not have the benefit of data from adequately designed and executed clinical trials, it did not require contraindication in patients without a gallbladder.”
Calling this number connects you with a Drugwatch.com representative. We will direct you to one of our trusted legal partners for a free case review.
Drugwatch.com's trusted legal partners support the organization's mission to keep people safe from dangerous drugs and medical devices. For more information, visit our partners page.


