Hip Replacement Risks, Complications & Device Failures
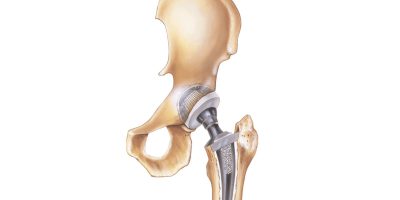
Hip replacements often improve mobility, but some fail due to loosening, infection or poor design. Understanding common complications can help you recognize warning signs and take action to protect your health and quality of life.
- Legally reviewed by Julie Lawson Timmer, Esquire
- Last update: April 23, 2025
Why Are Hip Replacements Failing?
Hip replacement surgery can significantly improve mobility and quality of life, but complications such as loosening, infection or dislocation sometimes occur. Poor implant design can contribute to early failure.
The Most Common Hip Replacement Complications
While most hip replacement surgeries go smoothly, there can be some complications. A 2022 review of joint replacements in the Annals of the Royal College of Surgeons of England found that about 5% of patients may face significant issues after surgery. This means that even though the surgery is often successful, roughly one in 20 people might experience hip replacement complications.
According to a 2024 analysis in the Archives of Orthopaedic and Trauma Surgery, the most common cause of total hip replacement revision surgery is aseptic loosening (35.1%), a condition in which the implant’s artificial components separate from the bone. Other common causes include deep infection (18.2%) and dislocation or instability (15.9%).
- Aseptic Loosening
- Aseptic loosening happens when a hip replacement becomes loose without infection, often leading to failure. It can occur because of poor initial attachment, the loosening of the implant from bone over time, or osteolysis (the destruction of bone tissue and resulting weakening of the bone) in the areas around the implant.
- Infection
- Infections can occur after surgery, either shortly thereafter (acute) or much later (chronic). These infections can cause sepsis, a sometimes deadly complication due to the body’s overreaction to an infection. Acute infections arise quickly from germs introduced during surgery, while chronic infections can develop years later from low-level pathogens. Treatment may involve antibiotics or, in severe cases, cleaning or replacing the implant.
- Instability and Dislocation
- A dislocation happens when the ball of a hip replacement comes out of its socket, leading to pain and limited movement. This can occur due to improper surgical techniques, individual health factors or failure to follow patient recovery guidelines. Repeated dislocations are more common after revision surgeries. Many can be treated non-surgically, but ongoing issues may require additional surgery.
In addition to these complications, fractures near the implant were responsible for more than 11% of revision surgeries.
How Poor Implant Design Leads to Early Failure
Poor hip implant design can lead to premature failure by creating mechanical weaknesses. Some designs concentrate stress in specific areas, weakening the surrounding bone and making failure more likely. Others don’t secure the implant properly, allowing it to shift over time. Some materials simply wear down too quickly, reducing stability.
- Stress Shielding: When implants are too stiff, they can create uneven pressure on the surrounding bone, leading to bone loss and loosening of the prosthesis over time. If manufacturers design implants poorly, they may fail to stimulate the bone properly, which is crucial for keeping the bone strong and healthy.
- Material Wear: When implants use materials that aren't ideal, they can break down and release tiny metal particles. This can lead to inflammation and damage to surrounding tissues, affecting the implant’s stability and lifespan. It's crucial to consider how these materials interact with the body, as this can lead to complications later on.
- Implant Migration: If a surgeon places a hip replacement implant insecurely or manufacturers shape it incorrectly, it can shift from where it should be. This movement, called implant migration, can cause early problems and is a common reason for hip replacement failure.
Metal-on-Metal Hip Implants
Metal-on-metal (MoM) hip implants have faced a turbulent journey since their introduction. Marketed as a lasting solution for younger patients, they became highly popular in the 1990s and made up over half of hip replacements in the U.S. by 2006.
However, issues arose when tiny metal particles released by the implants caused metallosis — a type of metal poisoning. European studies revealed that MoM implants failed two to three times more often than other types, leading to a decline in their use.
Surgeons in the United States no longer use metal-on-metal hip implants, although hip resurfacing — a process that coats the head of the thighbone with a metal surface — still occurs in rare instances. The Arthritis Foundation estimates that 1.5 million Americans still live with MoM devices or a resurfaced hip.
The Hidden Dangers of Metal-on-Metal Implants
Metallosis from MoM implants can lead to problems both near the implant and throughout the body. Locally, it can cause pain, swelling and discoloration of the surrounding tissue.
On a larger scale, these tiny metal particles can cause serious health conditions, including vision and hearing issues, memory problems, heart issues and thyroid complications. The immune system’s reaction to these particles also triggers inflammation, which can cause the implant to fail.
- Neurological and Cardiac Effects – Experts have linked metal particles in the bloodstream to vision loss, hearing issues, cognitive decline and heart problems.
- Treatment Challenges – The primary treatment for implant failure is currently revision surgery. But future research may explore chelation therapy (the use of chemicals to clear out heavy metals) to remove excess metal from the body.
If you have metal-on-metal implants, you need to be aware of these potential risks and consult with your health care provider if you experience any concerning symptoms.
Which Metal-on-Metal Hip Implants Have Been Recalled?
Hip replacement manufacturers have recalled thousands of defective devices. Hip replacement recalls often follow surges in complications reported to the company or the U.S. Food and Drug Administration.
- U.K.-based Smith & Nephew recalled all 6,266 of its Modular Redapt Hip Systems in 2016 because of “a higher than anticipated complaint and adverse event trend.”
- Smith & Nephew also recalled almost 6,000 Birmingham Hip Resurfacing components in 2015 because of “revision rates which were higher than established benchmarks.”
- Stryker recalled 9,003 ABG II Modular Hip Stems in April 2012 after reports of corrosion and fretting, a type of surface damage caused by friction between contacting surfaces. The junction where the device’s angled necks connected caused the shedding of particles.
- Johnson & Johnson subsidiary DePuy Orthopaedics issued a global recall for all ASR and ASR XL Acetabular Cup hip replacement components in 2010.
According to the FDA, if your hip implant is recalled, you may not need to replace it. Sometimes, a recall simply means you should monitor it more closely. It’s important to discuss any recalls with your surgeon to decide what to do next. Ask your orthopedic surgeon for advice if you’re unsure about your hip implant’s status.
How Does the FDA Approve Hip Implants?
The FDA regulates hip implants through two main methods: the premarket approval (PMA) process and the 510(k) clearance process.
The 510(k) process, known as the “fast-track,” allows companies to market their devices by proving they have “substantial equivalence” to existing devices. This method is mainly used for low- to moderate-risk devices and has faced criticism for its less strict requirements compared to the PMA process.
- Limited Safety Assurance: From 2003 to 2007, device makers put fewer than 1% of medical devices through the extensive human trials required for FDA approvals.
- Potential Risks: Without thorough testing on patients, there can be unexpected problems, as shown by the widespread MoM hip implant complications during the 2010s.
In 2016, the FDA established a new rule requiring premarket approval for all metal-on-metal hip devices. Today, no FDA-approved MoM total hip replacement devices exist.
Who Is Responsible for Defective Hip Implants?
Companies that make medical implants are responsible when their products are faulty. However, hospitals and doctors can also be responsible in certain situations.
When a medical device requires a recall, manufacturers often do so voluntarily to ensure public safety and avoid reputational damage. In rare cases where manufacturers fail to act on a harmful device, the FDA can intervene and issue a recall order.
Can Patients Hold Hip Implant Manufacturers Accountable?
People harmed by a defective hip implant can file hip replacement lawsuits against the implant’s manufacturer. Current hip replacement lawsuits include 1,851 cases in the Exactech multidistrict litigation (MDL) in a New York federal court. MDLs consolidate several similar cases into one litigation to make the legal process more efficient.
People filed Exactech lawsuits after the company recalled its medical implants in 2021 and 2022. The implants were shipped in packaging that allowed air to enter, causing them to break down early and requiring many patients to have surgery to replace them.
Attorneys filed tens of thousands of metal-on-metal hip replacement lawsuits in the 2010s. These were combined into multiple multidistrict litigations. Each MDL targeted a different manufacturer, with some companies involved in multiple MDLs related to their various devices.
In 2019, Johnson & Johnson agreed to pay $1 billion to resolve nearly 6,000 Pinnacle hip implant lawsuits in one of the largest and last MoM hip settlements.
What to Do if Your Hip Implant Is Failing
If you think your hip implant is failing, see your doctor immediately. They will perform a physical exam and may order imaging tests like X-rays, MRIs or CT scans to check for loosening or damage. Blood tests can also help detect infections or metal reactions. If any of these processes confirm that your implant is failing, you may require revision surgery.
How Can You Tell if Your Hip Implant Is Failing?
- Hip pain, especially while walking: Persistent or worsening pain can signal implant issues.
- Stiffness or difficulty walking: Reduced mobility may indicate loosening or wear.
- Instability: Feeling unsteady or experiencing difficulty balancing can be a warning sign.
- Grinding or clicking sounds: Noises from the implant may suggest mechanical failure.
- Swelling or inflammation: Chronic swelling could point to infection or metallosis.
- Repeated dislocations: Multiple hip dislocations suggest the implant may not be stable.
- Fractures near the implant: Breaks in the femur or pelvis around the implant may require revision surgery.
- Signs of infection: Fever, warmth, redness or drainage near the implant can indicate an infection.
If you have other health conditions, like heart disease, you might need to see a specialist to ensure you are healthy enough for surgery and recovery.
Calling this number connects you with a Drugwatch.com representative. We will direct you to one of our trusted legal partners for a free case review.
Drugwatch.com's trusted legal partners support the organization's mission to keep people safe from dangerous drugs and medical devices. For more information, visit our partners page.