Invokana
Invokana (canagliflozin) is a Type 2 diabetes medicine. It belongs to a class of drugs called sodium-glucose cotransporter 2 (SGLT2) inhibitors. Some of the rare, but characteristic side effects of Invokana include amputations, diabetic ketoacidosis (too much acid in the blood) and kidney injury.
Invokana is an oral medication used to treat Type 2 diabetes. It contains the active ingredient canagliflozin.
Invokamet and Invokamet XR are part of the Invokana family of medications. They are combination drugs containing canagliflozin and metformin.
These medications belong to a larger class of drugs called sodium-glucose cotransporter 2 (SGLT2) inhibitors.
Invokana and other SGLT2 inhibitors control blood sugar levels by causing excess sugar to leave the body through urine. This helps lower abnormally high blood sugar levels in people with Type 2 diabetes.
Studies show Invokana can cause the need for below-the-knee amputation. Other serious side effects include diabetic ketoacidosis, kidney injury, urinary tract infections and cardiovascular problems.
People who suffered Invokana side effects are suing the drug’s maker. Invokana lawsuits accuse Johnson & Johnson of failing to warn patients and their doctors of serious risks linked to the drug.
Invokana FDA Approval
In March 2013, the U.S. Food and Drug Administration approved Invokana as the first drug in its class.
The FDA approved Invokamet in 2014 and Invokamet XR in 2016. Invokamet contains regular release metformin, and Invokamet XR contains the extended release version of metformin.
Johnson & Johnson makes all three medications. The company made more than $1.4 billion off the medicines in 2016.
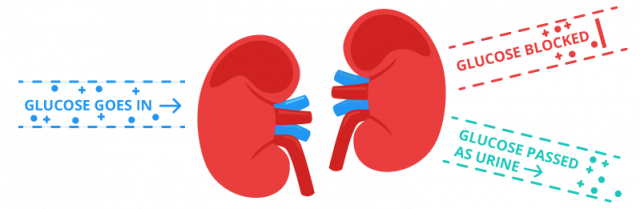
How Invokana Works
Invokana slows down the function of SGLT2. SGLT2 is a special protein in the kidney. It is responsible for putting 90 percent of the glucose from the urine back in the kidneys.
By slowing down SGLT2, Invokana allows more sugar to leave the body via the urine. This results in lowered levels of glucose in the blood.
Risks, Side Effects and FDA Black Box Warnings
2022 clinical studies link Invokana to rare but serious side effects. Some of these side effects of Invokana can be fatal.
Many side effects of Invokana require hospitalization, and intensive care or emergency treatment.
- amputations
- diabetic ketoacidosis (DKA)
- kidney injury
- urinary tract infections that lead to blood infections
- cardiovascular problems
- acute pancreatitis
- bone fractures
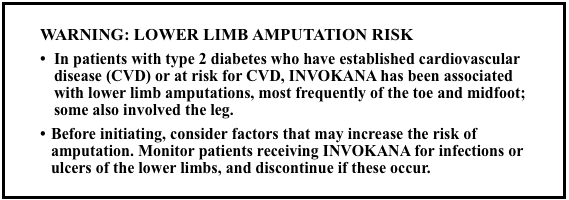
In 2017 the FDA required a boxed warning for an increased risk of leg and foot amputations with Invokana, Invokamet and Invokamet XR. This type of warning is the agency’s most serious warning. But in August 2020, the agency removed the black box amputation warning based on data from three clinical trials.
The amputation risk is still elevated with canagliflozin, but it’s “lower than previously described, particularly when appropriately monitored,” the FDA said in its 202o statement. Amputation warnings still remain in the Warnings and Precautions section of the prescribing information.
Invokamet and Invokamet XR also carry a black box warning for lactic acidosis.
Side effects that were less serious and more common in trials of Invokana included urinary tract infections and increased urination.
How to Take Invokana: Dosages and Recommendations
The medication insert recommends starting Invokana at a dose of 100 mg daily. Patients prescribed Invokana should take the drug before the first meal of the day.
If patients do not respond to 100 mg, doctors may prescribe 300 mg a day, according to the medication insert.


Patients with kidney problems should only take 100 mg of Invokana a day.
Invokana is not recommended in patients with serious kidney impairment who require dialysis.
Invokana Drug Interactions
Invokana may interact with some medications, according to the package insert. This means taking Invokana while you are taking other medications may make the drugs less effective. It can also cause unexpected side effects or increase the effects of a particular drug.
Patients should give their doctor and pharmacist a complete list of medications they are taking, including over-the-counter medications and herbal supplements, before starting treatment with Invokana.
- Rifampin, phenytoin, phenobarbital, ritonavir
- Taking Invokana with these drugs may decrease the effectiveness of Invokana.
- Digoxin
- Taking 300 mg Invokana with digoxin may increase the digoxin concentration in the blood.
- Other antidiabetic medications
- Taking Invokana with other antidiabetic medications may increase the risk of hypoglycemia (abnormally low blood sugar levels).
Contraindications: People Who Should Not Take Invokana
Invokana, Invokamet, and Invokamet XR are not meant for all diabetics.
The FDA has not approved the medicines for patients with Type 1 diabetes, or people with diabetic ketoacidosis (DKA) or a history of DKA. Patients with severe kidney problems should also avoid these medications.
If you have a history of a serious allergic reaction to canagliflozin, you should not use these drugs.
Pregnant Women
Invokana is not recommended during the second and third trimesters of pregnancy. Invokana affected kidney development in rats. This condition did not fully reverse within one month of a recovery period. There are no tests or data from pregnant women and risk cannot be ruled out. There are also risks to the mother and fetus associated with poorly controlled diabetes in pregnancy, according to the medication insert. Invokana, Invokamet, and Invokamet XR should only be used if their benefit outweighs their risks.
Nursing Mothers
It is not known if Invokana is excreted in human breast milk, but levels of Invokana were found in the milk of rats. Metformin from Invokamet and Invokamet XR may be present in human breast milk. There may be risk to the developing human kidney if babies ingest milk with traces of these drugs. Because of the potential for harm to infants, these drugs are not recommended for women who are breastfeeding. This advisement is included in the package insert. Before breastfeeding, mothers should talk to their doctors.
Use in Children
The safety or effectiveness of these drugs has not been tested in children under 18.
Seniors
People with Type 2 diabetes may have many treatment options available to them. Some doctors may prescribe insulin shots, pumps or injectors. It’s important to take them as prescribed.
All diabetes medications can cause side effects. Patients should discuss all options with their doctor before deciding on a treatment plan that works best for them.
Alternatives to Invokana
People with Type 2 diabetes may have many treatment options available to them. Some doctors may prescribe insulin shots, pumps or injectors. It’s important to take them as prescribed.
All diabetes medications can cause side effects. Patients should discuss all options with their doctor before deciding on a treatment plan that works best for them.
- Sulfonylureas
- Biguanides
- Meglitinides
- Thiazolidinediones
- DPP-4 inhibitors
- Alpha-glucosidase inhibitors
In addition to treatment with medications, certain lifestyle changes should be made as a part of any treatment plan for diabetes.
The most basic recommendation is to eat a healthy diet. A balanced diet can help patients maintain a healthy weight. It can also help patients better control blood pressure, cholesterol levels and blood glucose levels.
Doctors are likely to also recommend exercise or regular physical activity.
Calling this number connects you with a Drugwatch.com representative. We will direct you to one of our trusted legal partners for a free case review.
Drugwatch.com's trusted legal partners support the organization's mission to keep people safe from dangerous drugs and medical devices. For more information, visit our partners page.


