Xolair
Xolair (omalizumab) is an injectable prescription medication used to treat moderate to severe persistent allergic asthma and chronic hives (a condition called chronic idiopathic urticaria, or CIU). However, health complications in patients necessitated two FDA-approved label changes to the drug; one for anaphylaxis, which can be life-threatening, and another describing a slightly higher risk of heart and brain blood vessel problems, such as mini-strokes, heart attacks, chest pain, high blood pressure in the arteries of the lungs and blood clots.
Xolair (omalizumab) is an injectable medication used to treat patients with a moderate or severe type of asthma caused by allergens (allergic asthma) and a chronic type of hives with no identifiable cause (chronic idiopathic urticaria). But the drug has been linked to severe, life-threatening allergic reactions called anaphylaxis and other possible side effects.
A black box warning was added to the drug’s label in 2007, advising patients and physicians that anaphylaxis could occur at any time after taking Xolair.
In 2009, the U.S. Food and Drug Administration began evaluating safety findings of an ongoing study showing Xolair patients faced an increased risk of heart and brain blood-vessel problems.
The FDA finished its review of the study in 2014. While it found evidence of higher blood-vessel problems, there were flaws in the way the study was carried out. Due to these flaws, the FDA could not “definitively confirm or determine the exact increased level of these risks.” Slightly higher rates of cancer were also observed in previous Xolair studies. The FDA review did not find a difference in the rates of cancer, however, the flaws in the study also meant it couldn’t rule out the potential risk of cancer with Xolair.
The FDA initially approved the drug in 2003 for children 12 and older and adults. Novartis Pharmaceuticals Corporation and Genentech USA, Inc. announced in mid-2016 that the FDA had expanded Xolair users to include children as young as 6.
That made Xolair the first and only biologic treatment for patients 6 and older with uncontrolled moderate to severe persistent allergic asthma.
What Does Xolair Treat?
Xolair treats moderate to severe persistent asthma from allergies (allergic asthma) for patients 6 and older whose symptoms cannot be adequately controlled with inhaled corticosteroids.
Having allergic asthma means symptoms are caused by allergens, substances that trigger immune-system responses. The immune system releases immunoglobulin E.
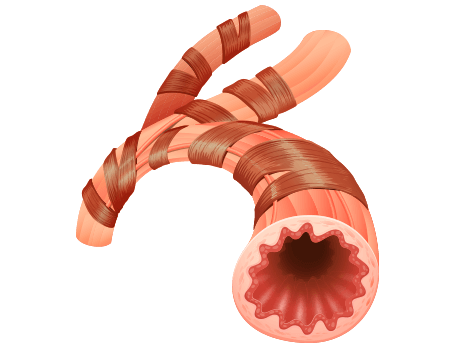
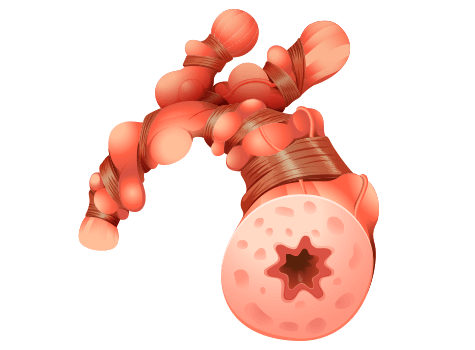
Too much immunoglobulin E can cause inflammation (swelling) in the body and in the airways in a person’s lungs. As the condition worsens, it can become difficult to breathe and may even result in an asthma attack.
Xolair blocks immunoglobulin E and may be a part of a treatment plan for people who suffer from allergic asthma.
What Is Asthma?
Asthma is a chronic disease that affects a person’s airways, causing them to swell, narrow and react strongly to allergens and other irritants. As the airways react and continue to narrow, less air can get through to your lungs.
- Animals (pet hair or dander)
- Cockroaches
- Changes in weather (usually colder temperatures)
- Exercise
- Pollen
- Certain medications (such as aspirin and other NSAIDs)
- Dust mites
- Strong emotions or stress
- Chemicals in the air or food
- Mold
- Respiratory infections (such as the common cold)
- Tobacco smoke
Asthma can lead to symptoms such as wheezing (a whistling sound when you breathe), coughing (especially early in the morning or at night), chest tightness and shortness of breath. If symptoms of asthma worsen, it’s called an asthma attack. Severe asthma attacks may require emergency care and can even be life-threatening.
Asthma affects people of all ages, but it most often begins in childhood. In the United States, more than 25 million people are living with asthma, and approximately 7 million of those people are children. There is no cure for asthma, but it can be managed with quick-relief and long-term medications, and symptoms can sometimes improve over time.
What Is Chronic Idiopathic Urticaria (CIU)?
Chronic idiopathic urticaria is the most common type of chronic hives. Xolair treats this condition in patients 12 and older who have persistent symptoms despite using anti-allergy drugs such as H1-antihistamines, or histamine blockers.
Urticaria is the medical term for hives, which are red and often itchy, sometimes painful, bumps on the skin that are usually caused by an allergic reaction to a drug or food. During an allergic reaction, the body releases chemicals called histamines that can make the skin swell into hives. Hives can also be caused by other conditions such as infections, stress and certain autoimmune conditions.
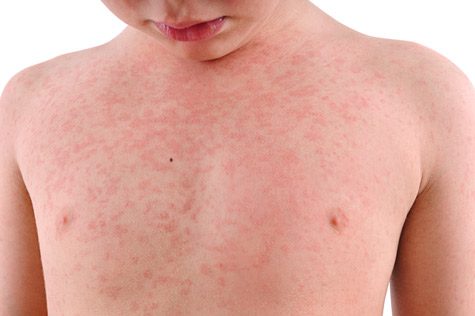
When hives last for six weeks or longer, the condition is considered chronic. In about three out of 10 cases, the cause of hives is known. But in seven out of 10 cases of chronic hives, a cause cannot be determined. This is diagnosed as chronic idiopathic urticaria (CIU), and it is the most common type of chronic hives, mostly occurring between the ages of 20 and 40. Women are more likely than men to have CIU, experiencing flares that last anywhere from one to five years or longer.
In rare cases, hives can cause swelling in a person’s airways making it difficult to breathe and requiring emergency treatment. If not immediately treated, this dangerous occurrence can even result in death.
How Is Xolair Administered?
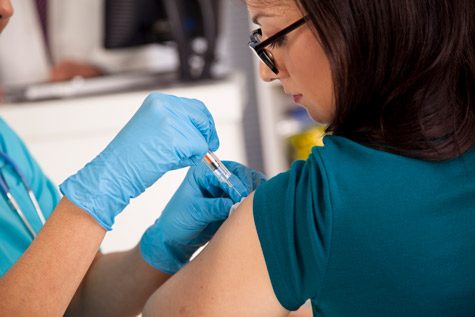
Xolair is an injectable prescription medicine administered by a health provider in a health care setting. It is a subcutaneous injection, meaning it is given in the fatty layer of tissue just under the skin. Subcutaneous injection sites can include the upper arms, abdomen in the belly area and front of the thighs.
As of 2022, the National Institutes of Health (NIH) advises rotating injection sites to keep the skin healthy, as repeated injections in the same location can cause scarring and hardening of tissues that can interfere with subsequent administration and absorption of medication.
Dosing Information
Xolair is administered in dosages of 150 milligrams to 375 milligrams every two to four weeks. Levels of immunoglobulin E and body weight are used to determine dosing and frequency. A patient’s physician should periodically reassess the need for continued treatment based on the severity of the disease and overall management of asthma symptoms.
When receiving Xolair for the treatment of allergic asthma, doses should be adjusted for any significant changes in body weight. Re-testing of immunoglobulin E (IgE) levels during treatment cannot be used as a guide for subsequent dose determinations and adjustments, due to the fact that total IgE levels are elevated as a result of treatment and will remain elevated for up to one year following the discontinuation of the asthma medication.
Dosing of Xolair in patients being treated for chronic idiopathic urticaria is not determined by IgE levels or body weight. Rather, treatments are administered by subcutaneous injection every four weeks in dosages of 150 milligrams or 300 milligrams. A patient’s physician should periodically reassess the need for continued treatment as the appropriate duration of Xolair therapy for chronic idiopathic urticaria has not yet been evaluated.
Xolair injections take approximately five to 10 seconds to administer due to the consistency of the solution being slightly viscous (thick and sticky). Each vial delivers 150 milligrams. If more than 150 milligrams is required, the doses need to be divided among two or more injection sites.
Overdosages
Studies have not yet determined the maximum tolerated dose of Xolair. When administering single doses of up to 4,000 milligrams to patients, no toxicity was reported. The highest cumulative dose administered to patients was 44,000 milligrams over a 20-week period with no adverse events or resulting toxicity associated with the higher dosing amount.
Side Effects of Xolair
Xolair is not without side effects, ranging from mild to severe, and even life-threatening. Anaphylaxis can occur after one dose or many doses. It can also occur immediately after receiving the injection or days later. If not immediately treated, anaphylaxis can be deadly. The FDA reviewed a study that found Xolair patients had a slightly increased risk of problems involving the heart and blood vessels in the brain. But the FDA could not determine a level of risk.
- Pain at the site of the injection
- Pain, especially in the arms and legs
- Feeling tired
- Bone fractures
- Dizziness
- Skin rash
- Pain or discomfort of the ears
Pediatric patients (ages six to 12) may experience slightly different side effects, including common cold symptoms, headache, fever, sore throat, earache or ear infection, abdominal pain, nausea, vomiting and nose bleeds.
- Nausea
- Swelling of the inside of your nose, throat or sinuses
- Joint pain
- Headaches
- Cough
- Upper respiratory tract infection
Injection site reactions include swelling, erythema (redness of the skin caused by increased blood flow in the capillaries in the lower layers of skin), pain, itching, bleeding and hives.
- Cancer
- Patients receiving Xolair may have a higher risk of certain types of cancer.
- Inflammation of blood vessels
- Symptoms include chest pain, shortness of breath, or a feeling of pins and needles or numbness of the arms and legs.
- Fever, muscle aches and rash
- These symptoms can occur one to five days after receiving Xolair.
- Parasitic infection
- This side effect can occur after receiving Xolair in patients who are at high-risk for parasite (worm) infections.
- High blood levels of a certain antibody (serum total IgE)
Drug Interactions
No formal drug interaction studies have been performed with Xolair. In patients with allergic asthma, the combined use of Xolair and allergen immunotherapy (commonly referred to as allergy shots) has not been evaluated. Similarly, it is unknown what interactions might occur in patients with CIU using Xolair and other immunosuppressive therapies simultaneously.
Adequate and well-controlled studies in pregnant women using Xolair have not been conducted, and therefore, the data is insufficient to inform patients on associated risk. Likewise, for breastfeeding mothers, it is unknown if Xolair is present in breast milk.
Xolair Lawsuits
In 2006, 2010 and 2012, Genentech and Novartis were hit with whistleblower lawsuits filed by Frank Garcia, Stephen Fauci and Allison Kelly, respectively, all former employees of the drug companies. Federal law allows whistleblowers to file suits on behalf of the government alleging fraud. If the government wins, whistleblowers receive a portion of the award.

With former employees accusing Genentech and Novartis of wrongdoing in the marketing and sales of Xolair, and with post-marketing studies and reports linking the use of Xolair to serious side effects, such as life-threatening anaphylaxis, heart attacks, mini-strokes, blood clots in the lungs, high blood pressure in the arteries of the lungs, and possibly even cancer, the drug companies may face lawsuits stemming from personal injury claims made by injured patients using Xolair.
The whistleblowers further claimed that Genentech and Novartis had misbranded Xolair “making it ineligible for reimbursement” under government health care programs, such as Medicare and Medicaid. Additionally, the whistleblowers claimed that health care providers were induced to submit claims for reimbursement at improper rates by using improper medical codes for the administration of Xolair.
But on June 17, 2016, the U.S. Court of Appeals affirmed a district court’s ruling to dismiss the federal claims with prejudice (meaning they cannot be amended, revised and refiled). The court found the whistleblowers failed to make their complaints with “sufficient particularity” (not clearly enough).
However, the appeals court found a procedural error and sent that part back to the district court, providing the whistleblowers an opportunity to amend and refile those claims.
Potential for Personal Injury Claims
With former employees accusing Genentech and Novartis of wrongdoing in the marketing and sales of Xolair, and with post-marketing studies and reports linking the use of Xolair to serious side effects, such as life-threatening anaphylaxis, heart attacks, mini-strokes, blood clots in the lungs, high blood pressure in the arteries of the lungs, and possibly even cancer, the drug companies are likely to face lawsuits stemming from personal injury claims made by injured patients using Xolair.
Law firms across the country are currently discussing legal options with injured consumers and preparing to file lawsuits on behalf of patients who have suffered serious side effects or even death after receiving Xolair treatments.
Calling this number connects you with a Drugwatch.com representative. We will direct you to one of our trusted legal partners for a free case review.
Drugwatch.com's trusted legal partners support the organization's mission to keep people safe from dangerous drugs and medical devices. For more information, visit our partners page.


