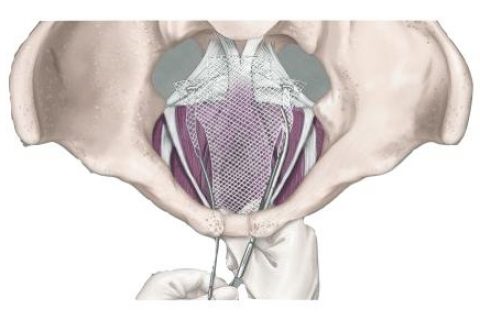
Transvaginal Mesh Complications: Pelvic Organ Prolapse
Complications from surgical mesh used in transvaginal pelvic organ prolapse (POP) repair include vaginal mesh erosion, pain and organ perforation. The Food and Drug Administration in 2016 reclassified mesh for transvaginal POP repair as a high-risk device and has said complications are “not rare.” In April 2019, the agency ordered all manufacturers to stop selling POP mesh because they had not proven its safety or effectiveness.
One review of several studies estimated that as many as one in four women implanted with mesh during transvaginal POP surgery may suffer complications.
After it had received more than a thousand reports of complications, the FDA in 2016 reclassified surgical mesh for transvaginal repair of pelvic organ prolapse (POP) as a Class III device.
The classification means mesh for transvaginal POP repair is among the riskiest medical devices. The most reported complication is vaginal mesh erosion, which occurs when the plastic, net-like device wears through vaginal tissue and becomes exposed.
“FDA has determined that the safety and effectiveness of surgical mesh for transvaginal POP repair has not been established and that the collection of additional clinical evidence on these devices is needed,” the agency said in its 2016 reclassification order.
The agency has been less critical of mesh bladder slings used to treat stress urinary incontinence. In general, mesh for POP repair has a higher complication rate.
In a July 2011 notice titled “Update on the Safety and Effectiveness of Transvaginal Placement for Pelvic Organ Prolapse,” the FDA concluded that “the risks of serious complications associated with transvaginal POP repair with mesh are not rare.”
Complications may happen immediately after surgery or many years later. Fiona Bicknell from the United Kingdom calls what she went through a “prolapse mesh nightmare.”
At first, the ProRepair mesh kit Bicknell received helped the symptoms of her prolapse, and she was very happy. Then, about 10 years later, serious complications turned her life upside down.
“Mesh has caused me catastrophic damage both physically and emotionally. I have been suicidal —chronic pain does that to you. I can’t have full sexual relations with the man I have loved for 16 years. I am no longer the fit, active, life-loving person I once was,” Bicknell told Drugwatch in an email.
Dr. Megan Schimpf, an associate professor of urology and obstetrics and gynecology at the University of Michigan, says some complications with mesh surgery may occur because surgeons don’t have proper training.
“We don’t want to get the message out there that all mesh is bad, or that mesh has to be removed simply because other women are having problems,” Schimpf told Michigan Health in May 2018. “Many women are happy and symptom-free after mesh surgery.”
Still, the implant remains controversial. Thousands of transvaginal mesh lawsuits filed in the United States and criticism from the FDA have led doctors and patients to question the device’s safety. Seven manufacturers have paid out nearly $8 billion to over 100,000 women, Carl Heneghan reported in his June 2019 article in the BMJ.
Safety concerns led the FDA to stop all sales and distribution of POP mesh in the United States on April 16, 2019.
In June 2022, Boston Scientific published the results of its 522 safety and effectiveness study required by the FDA Obstetrics and Gynecology.
“Transvaginal mesh repair for the treatment of anterior and/or apical vaginal prolapse was not superior to native tissue repair at 36 months,” researchers found. They also found transvaginal mesh was similar in safety to native tissue repair for POP.
How Often Do Complications Occur?
Exact numbers may vary depending on the study conducted, but according to a review of a wide variety of studies by D. Barski and D.Y. Deng in BioMed Research International, the complication rate for mesh for transvaginal POP repair is about 15 percent to 25 percent.
Some studies have found lower complication rates. For example, in a Canadian study published by the International Continence Society, researchers Aube and colleagues said they found complication rates lower than those the FDA had found.
The FDA reported 10 percent of patients suffered vaginal extrusion versus 2.6 percent in the Canadian study. Up to 50 percent suffered from painful intercourse in rates reported by the FDA versus less than 2 percent in the Canadian report.
The same authors reported in a separate 2018 study that 37 patients out of 334 required repeat operations. Of those women, 17 had recurrent prolapse and 10 suffered mesh exposure.
Problems Reported to the FDA
In a January 2012 article in OB.GYN. News, Dr. Andrew I. Brill summarized POP adverse events submitted to the FDA’s Manufacturer and User Facility Device Experience (MAUDE) database from 2005 to 2010. He told the publication he was a consultant and speaker for Ethicon Endosurgery, Gynecare, Conceptus and Karl Storz.
Brill’s summary ranked the complications from 1 to 10, with erosion as the most reported issue followed by pain and infection. While the ranking included the problems most commonly reported to the FDA, women may experience other issues. For example, Bicknell told Drugwatch she suffers hip pain and urethral burning.
| Complications | Number of Reports |
|---|---|
| Erosion | 528 |
| Pain | 472 |
| Infection | 253 |
| Bleeding | 124 |
| Dyspareunia (painful sex) | 108 |
| Organ perforation | 88 |
| Urinary problems | 80 |
| Vaginal scarring | 43 |
| Neuro-muscular problems | 38 |
| Recurrence | 32 |
By Jan. 31, 2019, MAUDE had received 139,000 adverse event reports related to gynecological mesh implants. Of these, 69,000 were for POP mesh, and 393 patients died, according to data presented by CEO of Device Events and former FDA consultant Madris Tomes.
Removal of mesh, also known as revision surgery, is often the only solution to these issues, but removal is not always possible. Some doctors compare mesh removal to cutting gum out of hair. In many cases, multiple surgeries are required, and there is no guarantee that surgeons can remove all of the mesh.
Of the general mesh complications reported, erosion, pain and organ perforation are some of the more difficult to treat.
Vaginal Mesh Erosion Is Most Common Complication
Erosion of mesh through the vagina, also called exposure, extrusion or protrusion, is the most commonly reported complication of transvaginal POP repair with surgical mesh. It occurs when transvaginal mesh erodes through tissue and becomes visible. Erosion can be extremely painful, and vaginal erosion usually makes sex too painful to have.
Erosion can affect several other organs besides the vagina. Urinary tract erosion can cause recurring infections and severe pain. Erosion into the bladder or rectum can cause infections, pain and abnormal connections known as fistulas.
- Abscesses (pus-filled sores)
- Vaginal discharge
- Vaginal scarring
- Neuro-muscular problems
A 2013 review by David R. Ellington and Holly E. Richter published in Clinical Obstetrics and Gynecology reported previous studies had found erosion occurred in about 10.3 percent of women who received mesh for transvaginal pelvic organ prolapse repair. Other studies have found that erosion rates vary from 7.3 percent to 21 percent, according to a 2012 review published by Drs. Hemendra N. Shah and Gopal H. Badlani in the Indian Journal of Urology.
In a 2017 study by Cheng and colleagues published in the Taiwanese Journal of Obstetrics and Gynecology, authors found 47 out of 741 women who underwent mesh repairs suffered erosion. They found women who had hysterectomies and/or hypertension had a greater risk of erosion.
Some doctors have blamed the high rate of erosion or exposure on inexperienced surgeons. A 2015 study by Illston and colleagues looked at the rates of these complications in women who received the Prolift mesh kit implanted by an experienced surgeon. Out of 160 women who completed a survey, 23 had confirmed mesh exposure.
One of the other reasons mesh erosion is such a serious complication is that the rate of occurrence increases over time. For example, a 2018 study by Thierry Vancaillie and colleagues found the erosion rate was 17 percent at one year and it jumped to 42 percent at seven years.
Pain After Mesh Implantation
Pain is one of the biggest problems women face after receiving mesh. Ellington and Richter’s review found women reported pain following a mesh procedure in 70 out of 110 studies. Overall, the rate was 9.1 percent. That’s about nine people for every 100.
In one study included in the review, 23 women had mesh removal surgery for complications, and out of 11 women who complained of pain, 10 found relief from pain after the mesh removal.
“I had sharp stabbing pains vaginally that knocked me off my feet. I felt like I had been kicked hard between the legs.”
Ellington and Richter found another observational study that showed the rate of pain during sex was 20 percent. But the researchers also found a study showing patients who had non-mesh repairs reported more pain during sex than those in the mesh group.
While some women have uncomfortable symptoms from POP before mesh, many women report new pelvic pain — called de novo pain — after the surgery.
“I had sharp stabbing pains vaginally that knocked me off my feet,” Bicknell said of her de novo pain. “I felt like I had been kicked hard between the legs.”
In a 2017 study by Elizabeth J. Geller and colleagues, 15.6 percent of 160 women reported new onset pain at least a year after surgery. That equates to one in six women, the authors wrote. Only about half of the women were sexually active a year after surgery and 19 percent reported painful intercourse.
Geller and colleagues found risk factors for pain after surgery included younger age, early post-operative pain, fibromyalgia and poor physical health.
The immune system might also play a role in pain symptoms, according to a 2019 study by Dr. Lauren Tennyson and colleagues published in the American Journal of Obstetrics and Gynecology. Researchers found women with mesh had higher levels of T cells and collagen buildup. This makes the tissue less flexible and causes it to pull on nearby muscles and other body parts, leading to pain.
Perforation of Multiple Organs
Organ perforation is defined as the penetration of the wall of an organ in the body. As mesh erodes through internal tissues, it can perforate other organs. This usually happens to the bladder, urethra, bowel or rectum. Doctors consider this one of the most serious complications of mesh surgery because of the risk of infection and organ damage.
Altman and colleagues studied 389 women with pelvic organ prolapse in a 2011 study comparing transvaginal mesh procedures to colporrhaphy, a surgery to repair the vaginal wall. Two hundred women underwent transvaginal mesh repairs, and 189 underwent colporrhaphy. All of the women had a prolapse in the anterior (front) vaginal wall.
Complication rates were significantly higher for the mesh group, particularly for perforation. These women suffered a 3.5 percent incidence of bladder perforation versus only 0.5 percent in the colporrhaphy group.
On rare occasions, perforation occurs during implantation. This happens when surgical tools puncture the bladder, bowel or blood vessels. One study by Caquant and colleagues followed 684 women after mesh surgery. They found five women had bladder wounds and one had a rectal wound following surgery.
Removing mesh is the first treatment for mesh that is cutting through organs. Surgeons may reconstruct mesh-damaged tissue or cauterize (burn) ruptured blood vessels to stop bleeding. If urine or feces leak from the bladder or bowels, doctors remove the waste using a catheter or other devices. If perforation causes infection, antibiotics are prescribed. Removing mesh may require multiple surgeries.
Minimizing Complications
Dr. Gopal H. Badlani, professor and vice chair of urology at Wake Forest School of Medicine, said training in doing the procedure and selecting the right patient are key to decreasing the rate of complications.
“Once you do that, then of course you have less chance of complications, but it will never be zero,” Badlani told Drugwatch. “So the patient must understand that.”
Mesh repairs are not recommended for everyone. Patients who’ve had prior radiation to the area should not undergo repairs with mesh. Patients who smoke have a higher risk of exposure than patients who do not smoke. Someone who takes steroids has a high risk of exposure of the mesh in the vagina compared to someone who does not take steroids. And simultaneous hysterectomy has a higher rate of exposure.
“So these are host factors and then there is the technique factor and finally the size of the mesh you’re using and you’re incision size,” Badlani said.
He said the bigger the incision and the larger the piece of mesh, the greater the rate of exposure. He also said centers that only perform mesh procedures occasionally will have a higher rate of complications compared to centers that perform a large number of the surgeries.
“So if you take a sling, which is a very narrow strip of the mesh, and a small incision in the vagina, the exposure rate in large theories is less than 1 percent, overall it’s less than 3 percent. But if you see centers that do 100 cases a year, you’ll see the exposure rate to be less than 1 percent,” Badlani said. “There are people who are fellowship-trained in these procedures versus people who attend a weekend course and start doing it or people who watch it on a video and then start doing it. So there’s a different level of training that does play a role.”
Unlike other medical devices, transvaginal mesh kits were not tracked when doctors first started implanting them in patients.
“Subsequent to the FDA warning now there is tracking by the companies of these kits, so you have a better sense of who’s using it, how often are they using it and what’s the rate of complications, so you can pick out an outlier and that means if some center is having much higher rate of complications then you need to go back and see what are they doing that could be adding more complications,” Badlani said.
Conditions that Mimic Mesh Complications
Mesh complications such as pelvic pain, bleeding and urinary problems can mimic other conditions, such as: Ovarian cancer, uterine fibroids, endometriosis, ovarian cysts and a painful bladder condition such as interstitial cystitis.
Pudendal nerve entrapment, also known as pudendal neuralgia, manifests as chronic pelvic pain. Pelvic surgery such as mesh surgery to repair pelvic organ prolapse is one of the most common causes of pudendal neuralgia. If mesh has to be removed, the incidence goes up, according to Drs. Jasmeen Kaur and Paramvir Singh.
Because of the pelvic pain, some women reported being diagnosed with interstitial cystitis. IC is typically treated with an oral medication called Elmiron.
Elmiron’s most common side effects include headache and diarrhea, but recent studies have linked the drug to a degenerative eye problem called pigmentary maculopathy. Pigmentary maculopathy could lead to permanent vision loss.
To avoid unnecessary treatment for other conditions, patients should ask their doctors to rule out mesh complications including pudendal neuralgia.
Calling this number connects you with a Drugwatch.com representative. We will direct you to one of our trusted legal partners for a free case review.
Drugwatch.com's trusted legal partners support the organization's mission to keep people safe from dangerous drugs and medical devices. For more information, visit our partners page.