Bladder Sling Complications
Bladder sling surgery is a common treatment for stress urinary incontinence, but it can come with risks. Most women tolerate the procedure well but can experience mesh complications, erosion and organ perforation. Complications can occur during surgery or years later.
Types of Bladder Sling Complications
Women receive bladder slings to treat stress urinary incontinence, but these slings can have many risks. Some may not meet the criteria for surgical complications according to traditional definitions. These typically develop after hospital discharge because a routine surgical audit did not identify them.
Several countries have banned transvaginal mesh products, including some types of slings, because of complications. Mesh sling procedures can result in problems such as bladder perforation, mesh erosion into the vagina and painful intercourse. Women who have suffered complications are calling for a ban on these devices, and some have filed transvaginal mesh lawsuits.
- Bladder outlet obstruction
- Bleeding
- Infection
- Intestinal perforation
- Pain
- Urinary tract infection
- Urinary urgency
- Vaginal extrusion of mesh
Unfortunately, these injuries can cause recurrence or even worsen urinary problems. Despite these risks, most doctors prefer mesh slings made of polypropylene, a type of plastic, to treat stress urinary incontinence. The U.S. Food and Drug Administration and doctors agree that bladder slings are less problematic than mesh for treating pelvic organ prolapse. In January 2016, the FDA reclassified transvaginal mesh as a high-risk device.
Most Common Bladder Sling Complications
Mesh slings are more likely to cause complications than slings made of native tissues. This is because synthetic mesh can cause many problems, including infection and long-term pain. According to the American College of Obstetricians and Gynecologists, these risks are either not present or greatly reduced.
Pain is one of the most common bladder sling complications, with pain reported to persist in 42.3% of patients who underwent revision surgery. In addition, there is an up to 13% chance of potentially recurring urinary tract infections. Other pervasive risks are mesh erosion and urinary retention issues.
- Bleeding
- Erosion through the urethra
- Fatigue
- Fistula formation
- Infection
- Inflammation
- Intestinal perforation
- Local irritation at wound site
- Mesh erosion
- Migration of the device
- Nerve damage
- Pain
- Recurrence of incontinence
- Scar contracture
- Scarring
- Shortness of breath
- Swelling and redness at the wound site
- Urinary tract obstruction and urine retention
- Vaginal discharge
- Vaginal extrusion
It is important to note that all surgeries carry some risk of complications. If you are experiencing any of the above symptoms, be sure to reach out to your doctor to talk about the best course of action moving forward.
Bladder Sling Complications and Interstitial Cystitis
Bladder sling complications sometimes resemble symptoms of interstitial cystitis. IC is a painful bladder condition that affects millions of Americans, more often women than men. Common symptoms of IC that may also be symptoms of bladder sling complications include pelvic and bladder pain, painful sexual intercourse and urinary urgency. It is important to note that doctors can often misdiagnose pudendal neuralgia as IC, so women diagnosed with either should discuss this with their medical provider.
While surgery may treat bladder sling complications, medications typically treat IC. The only FDA-approved oral drug to treat IC in the United States is Elmiron (pentosan polysulfate sodium). However, some recent studies have linked Elmiron to a degenerative vision condition called pigmentary maculopathy.
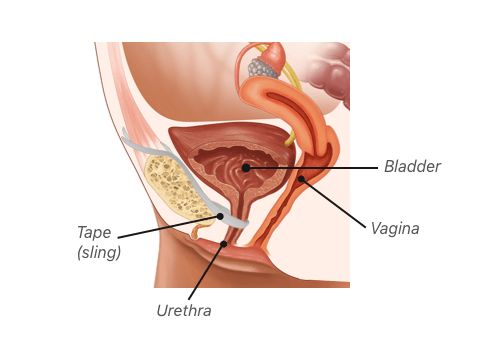
Bladder Sling Perforations
Bladder and bowel perforation after mesh placement can be serious and lead to infections and other problems. Bladder perforation occurs when the mesh or surgical tools puncture the bladder during surgery. This can cause pain, bleeding and urinary tract infections. In most cases, bladder perforation does not cause long-term injury. However, it can sometimes require surgery to repair the bladder.
A punctured bowel is a more severe type of perforation. It can cause life-threatening infections and sometimes require surgery to remove the mesh and repair the bowel.
“Intraoperative bladder perforation was associated with younger patient age and lower BMI. Additionally, bladder perforation is a risk factor for postoperative urinary tract infection and lower urinary tract symptoms.”
Source: Urology
In a study published in Urogynecology, 8% of patients experienced bladder perforation. Risk factors for perforation were having a body mass index less than 30 and a history of a previous hysterectomy.
The treatment for bladder and bowel perforations depends on the severity of the injury. In some cases, the perforation may be small enough to heal on its own with antibiotics. In other cases, surgery may be necessary to repair the perforation and remove the mesh. Surgeons can perform mesh removal laparoscopically or through a larger incision in the abdomen.
Vaginal Extrusion and Erosion From Bladder Slings
Mesh extrusion and erosion are two of the main concerns with bladder slings. Extrusion refers to the mesh forcing its way out of the vagina, while erosion refers to the mesh wearing through the vaginal wall and into the vagina. The risk of mesh extrusion and erosion varies depending on the type of sling used. Retropubic slings have a lower risk of vaginal erosion than transobturator slings. However, both types of slings can cause deterioration, which can happen several years after surgery.
- Retropubic Slings: Extrusion rates vary from 0% to 1.5%
- Transobturator Slings: Extrusion rates range from 0% to 10.9%
- Urethral Erosion: Happens after less than 1% of sling surgeries
An exact cause of mesh extrusion and erosion has not been determined. The main theory is that the mesh may irritate the vaginal wall, causing it to break down. The mesh may also move out of position over time, leading to erosion.
If you suspect you have bladder erosion, it is vital to seek medical attention as soon as possible. Bladder erosion is a serious complication that can cause pain, bleeding and infection and may require surgical removal.
Diagnosing Bladder Sling Complications
Bladder sling complications can be challenging to diagnose, so it’s crucial for doctors to carefully examine the patient to understand the location and extent of the mesh placement. Pelvic structures that can be injured during or after mesh surgery include the sacrum, bladder and rectum.
According to the American College of Obstetricians and Gynecologists, diagnostic testing can include cystoscopy, proctoscopy, colonoscopy or radiologic imaging. Because of the number of variants involved, there are no universal medical recommendations for minimum testing to diagnose.
Treating Bladder Sling Complications
Bleeding, short-term urinary retention and pain are usually minor complications of bladder sling surgery and are easier to treat. Long-term complications can occur years after surgery and include vaginal extrusion, erosion, organ perforation and recurrent infections.
Pain medication, antibiotics or minor surgery can often treat short-term complications. Physiotherapy and psychological and psychosexual support can also be very effective. More severe complications will require transvaginal mesh removal surgeries, which can come with risks. It is possible that mesh removal does not improve symptoms and may cause infection, bleeding or issues with anesthesia.
Calling this number connects you with a Drugwatch.com representative. We will direct you to one of our trusted legal partners for a free case review.
Drugwatch.com's trusted legal partners support the organization's mission to keep people safe from dangerous drugs and medical devices. For more information, visit our partners page.

