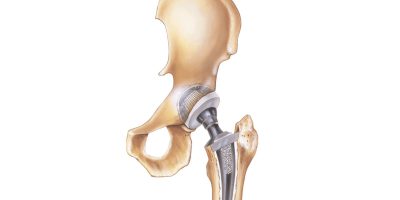
Hip Replacement Lawsuits
Hip replacement lawsuits are being filed by people facing serious complications from their hip replacement devices. Lawyers have filed thousands of lawsuits against hip manufacturers, including Stryker and DePuy. Companies have paid billions in settlements and verdicts.
- Legally reviewed by Tess Schulman, Ph.D.
- Last update: June 13, 2025
Who Qualifies for a Hip Replacement Lawsuit?
If you suffered an injury from a hip implant, then you may qualify for a hip replacement lawsuit. Common injuries listed in lawsuits have included metal poisoning, tissue damage, severe pain, implant failure and revision surgery.
Most large hip replacement actions involve metal-on-metal designs. But other types of hip replacement lawsuits include complications related to parts of hip replacement systems like polyethylene hip liners.
“There’s a lot of different brands [involved in lawsuits]. A lot of times people won’t know their manufacturers, or they won’t know their model, and generally you have to know both of them … That’s another reason why you should speak to a lawyer, because we’re happy to get those medical records, take a look at the product itself and see if you had one of those defective products,” attorney Daniel Nigh told Drugwatch.
Did you experience serious complications from your hip replacement device?
Get Your Free Case Review
A type of osteolysis or bone damage can result from joint replacement surgery. As the Hospital for Special Surgery explains, “Occasionally, polyethylene or other materials in a joint implant can wear down. When this happens, debris can accumulate in the surrounding joint tissue. This, in turn, causes inflammation that can result in degeneration of the bone.”
Lawsuits have recently been filed, for example, as a result of premature wear of Exactech GXL liners, for example. In June 2021, the company notified surgeons that issues had been observed and the GXL liners had been transitioned entirely out of the US market. Impacted patients have begun filing lawsuits to obtain compensation for medical expenses, pain and suffering.
Since 2002, hip manufacturers have paid more than $7.5 billion to settle thousands of hip lawsuits. Recent jury verdicts against manufacturers have totaled more than $1.7 billion. But courts have tossed out or reduced many of those.
While most lawsuits involving metal-on-metal hip implants have been resolved, more than 2,173 hip cases — out of a total of 33,450 filed — were still pending across the country as of June 2025.
Lawsuits over Stryker’s LFIT V40 femoral head and Smith & Nephew’s BHR and R3 hips have been consolidated in federal and state courts. And law firms have just begun evaluating cases involving Stryker’s Tritanium Acetabular Shells, which are prone to loosen and fail.
Metal-on-metal hip replacement designs were supposed to be more durable, but lawsuits claim the devices shed microscopic amounts of chromium, cobalt or other metals into the body.
According to complaints, the design flaw caused a condition called metallosis, which destroys bone, muscle and other tissue. In addition to creating immediate health problems for the patient, the condition can weaken an implant and cause it to fail.
Lawsuits say several other complications occurred as a result.
- Metallosis
- Pain
- Dislocation
- Loosening
- Revision Surgery
Metal-on-metal implants became popular throughout the 2000s but quickly fell out of favor as reports of complications mounted. This led to hip replacement recalls, lawsuits and FDA actions. In 2016, the FDA strengthened the approval process for metal-on-metal hips.
Not all metal-on-metal hip models with high complication or failure rates were recalled. Manufacturers simply removed some of them from the market. DePuy’s Pinnacle hip was never recalled. But the largest jury verdicts in hip lawsuits have involved Pinnacle hips.
Hip Replacement Lawsuit Updates
As of June 2025, many of the companies involved in hip replacement lawsuits have settled cases for undisclosed amounts and have faced millions of dollars in jury verdicts. Several of the multidistrict litigations that once contained tens of thousands of lawsuits have been resolved and some have been closed or will soon be closed.
However, lawyers are still accepting hip implant cases for settlement negotiations even as they prepare for trial. One group of lawsuits against Exactech for its MCS, Acumatch & Novation GXL Liners is in the bellwether test trial selection process.
DePuy Orthopaedics
As of June 2025, DePuy has settled about 99% of its hip replacement lawsuits. The U.S. Judicial Panel on Multidistrict Litigation combined the federal lawsuits in two separate litigations, known as MDLs. Settlements and jury verdicts in DePuy hip lawsuits total more than $6 billion.

Device: DePuy Pinnacle Hip Replacement
- Number of Lawsuits: 10,581 total filed with 0 pending as of June 2025
- Litigation Location: Northern District of Texas
- Verdicts: March 2016 – $502 million (thrown out on appeal, retrial expected)
December 2016 – $1 billion (reduced to $543 million)
November 2017 – $247 million (reduced to $245 million) - Status of Lawsuits: Settled or dismissed
Device: ASR Hip Replacements
- Number of Lawsuits: 10,453 total filed with 139 pending as of June 2025
- Litigation Location: Northern District of Ohio
- Settlements: 9,800 settled for $4.42 billion between 2013 and 2015
- Status of Lawsuits: Many settled, but the MDL remains active
Smith & Nephew
As of June 2025, MDL 2775 remains open but with only 1 case still pending. In October, the MDL judge issued an order that closed the MDL to new filings. It is only currently open for administrative matters. The few remaining cases may be sent back to the courts they were initially filed in if they remain unresolved.
The U.S. Judicial Panel on Multidistrict Litigation grouped the first Smith & Nephew lawsuits into a single litigation in 2017.

Devices: BHR and R3 Hip Implants
- Number of Lawsuits: 1,089 total filed with 1 pending as of June 2025
- Litigation Location: District of Maryland
- Verdicts/Settlements: No verdicts or major settlements yet
- Status of Lawsuits: Active, some cases have been dismissed and no global settlement has been reached
Stryker
As of June 2025, Stryker hip lawsuits are still active for the company’s LFIT Anatomic CoCR V40 femoral head and Rejuvenate and ABG II hip implant. Lawsuits over the Rejuvenate and ABG II hips were transferred to the same court. Most of those cases were settled in 2014.
More recently, the U.S. Judicial Panel on Multidistrict Litigation created a single litigation, or MDL, for cases involving Stryker’s LFIT V40 hip component. Stryker agreed to an initial settlement in those cases in November 2018. Law firms have also accepted cases involving Stryker’s Tritanium Acetabular Shells.

Device: LFIT V40 Femoral Head
- Number of Lawsuits: 1,234 total filed with 138 pending as of June 2025
- Litigation Location: District of Massachusetts
- Verdicts/Settlements: Initial settlement announced Nov. 2, 2018, details remained confidential
- Status of Lawsuits: Currently in the settlement phase, MDL remains open
Device: Rejuvenate and ABG II Hip Replacements
- Number of Lawsuits: 3,637 total filed with 51 pending as of June 2025
- Litigation Location: District of Minnesota
- Settlement: $1.4 billion between 2014 and 2016
- Status of Lawsuits: Settled, but the MDL remains active
Tritanium Acetabular Shells
- Number of Lawsuits: Unknown (firms are evaluating cases)
- Litigation Location: To be determined
- Settlement: None
- Status of Lawsuits: Early phase
Zimmer
In July 2024, Zimmer Biomet issued a voluntary recall of its CPT Hip System because of an increased risk of thigh bone fractures. The company also said that it would stop selling this device by December 2024. Lawyers are currently investigating and accepting new cases tied to this device.
In March 2016, Zimmer offered to settle its Durom Cup lawsuits for $314 million. In May of that year, a court ordered that everyone with a case in the multidistrict litigation had to participate in the settlement process.
In November 2018, a federal panel combined 21 new lawsuits over other components into an MDL in New York. As of June 2025, lawyers are accepting Zimmer lawsuits for models of the company’s M/L Taper Hip Prosthesis used with Zimmer’s Versys Femoral Head.

Device: Durom Cup
- Number of Lawsuits: 750 total filed
- Litigation Location: District of New Jersey
- Settlement: $314 million to settle all cases
- Status of Lawsuits: Settled and the multidistrict litigation is closed
Device: Zimmer M/L Taper Hip Prosthesis and Versys Femoral Head
- Number of Lawsuits: 2 remain pending of 332 total filed as of June 2025
- Litigation Location: Southern District, New York
- Settlement: Zimmer offered to settle cases in 2023 for an undisclosed amount
- Status of Lawsuits: Settlement talks, MDL remains open
Biomet
As of June 2025, lawyers are still accepting individual Biomet hip implant lawsuits though the original MDL was closed after Biomet settled thousands of lawsuits when it merged with Zimmer.
A settlement in more than 2,800 Biomet hip lawsuits came as the company merged with Zimmer in 2015. Zimmer absorbed some of the costs of settling the lawsuits, which had been combined in a multidistrict litigation.
Biomet was purchased by Zimmer in 2015.

Device: M2a Magnum Hip Replacement
- Number of Lawsuits: 2,883 total filed
- Litigation Location: Northern District of Indiana
- Settlements: Estimated $89.4 million between 2013 and 2015
- Status of Lawsuits: Settled and MDL closed in September 2022
Wright Medical
As of June 2025, some lawyers are still accepting individual Wright hip lawsuits even if the majority of the cases were settled in 2017 and most payments were sent in 2019. At one time, the company faced about 2,000 lawsuits in multidistrict litigation.
After losing two bellwether trials, Wright agreed to settle the remaining hip implant lawsuits in 2017 for $90 million. In 2019, it settled roughly 1,200 lawsuits for $240 million.

Devices: Conserve, Dynasty and Lineage Replacements and Related Components
- Number of Lawsuits: 640 total filed
- Litigation Location: Northern District of Georgia
- Settlements: $330 million between 2016 and 2017
- Status of Lawsuits: Settled, MDL closed in November 2022; court ordered no new lawsuits may be filed
Exactech
Injured patients are still filing Connexion GXL hip liner lawsuits in June 2025. In December 2023, plaintiffs and defendants in the New York MDL as well as the Exactech litigation in Florida began the process of bellwether test trial selection. Next, they will develop a bellwether plan for the MDL cases.
Exactech established a claims process in 2021 for patients with premature wear and osteolysis as a result of the polyethylene liners in their hip replacement systems. But that didn’t stop patients from filing lawsuits that claimed the company produced a faulty product and failed to warn the public of the risks.

Devices: MCS, Acumatch & Novation GXL Liners
- Number of Lawsuits: 1,842 pending of 1,851 total filed as of June 2025
- MDL Litigation Location: Eastern District of New York
- Settlements: None yet
- Status of Lawsuits: Individual cases are being investigated and parties are selecting bellwether test trial cases
Timeline of Metal-on-Metal Hip Recalls and Lawsuits
-
2000
Sulzer Orthopedics recalls 40,000 hip replacement sockets for dislocation risk.
-
2001
More than 2,200 people require revision surgery to replace Sulzer implants.
-
2001
Three women win a $15.4 million verdict against Sulzer. The number of lawsuits filed reaches 1,000.
-
2002
Sulzer settles 4,000 lawsuits for $1 billion ($200,000 to each plaintiff).
-
2008
Zimmer temporarily recalls its Durom Cup hip component.
-
2010
The U.S. Judicial Panel on Multidistrict Litigation consolidates Durom Cup lawsuits into a multidistrict litigation.
-
2010
The judicial panel combines DePuy ASR lawsuits into an MDL.
-
2011
Lawsuits over DePuy’s Pinnacle hip implants are combined in an MDL.
-
2012
Wright Medical, Smith & Nephew and Biomet each issue extensive hip replacement recalls.
-
2012
Lawsuits over Wright Medical’s Conserve implants are combined into an MDL.
-
2012
Lawsuits over Biomet’s M2a Magnum implants are combined into an MDL.
-
2013
The judicial panel combines lawsuits over Stryker’s Rejuvenate and ABG II into an MDL.
-
2016
FDA strengthens approval process for metal-on-metal hips.
-
2016
Stryker recalls more than 42,000 LFIT V40 femoral head components.
-
2017
The judicial panel combines lawsuits over Smith & Nephew BHR and R3 implants into an MDL.
-
2017
The MDL panel combines the first federal lawsuits over Stryker LFIT V40 femoral heads into an MDL in Massachusetts federal court. New Jersey follows suit a month later, combining state lawsuits over the device into an MCL in state court.
-
2018
A federal judge in Atlanta closes the MDL involving Wright Medical hips following a 2017 settlement.
-
2018
Federal panel creates MDL for Zimmer’s M/L Taper Hip Prosthesis including versions with Kinectiv Technology and Versys Femoral Head.
-
2018
Stryker agrees to an initial settlement in the LFIT V40 MDL, but details are kept confidential.
Questions Your Hip Replacement Lawyer May Ask
What complications are you experiencing from your hip implant?
Patients usually experience relief from pain or increased mobility after recovering from their hip replacement surgery, but sometimes, complications may occur. Complications may range from dislocation and change in leg length to bone fractures and metallosis.
When did you first start experiencing hip replacement complications?
Hip replacement complications can occur during surgery, immediately following the procedure or years later.Let your hip implant lawyer know when you first noticed complications.
When did you have your hip replaced?
Knowing when you had your hip replacement procedure could help your hip injury attorney determine whether you’re within the statute of limitations to file a lawsuit.
What company made your hip replacement device?
Lawsuits have been filed against DePuy, Stryker, Zimmer, Smith & Nephew, Biomet and Wright. You can contact the surgeon who performed your procedure if you don’t know who made your hip implant.
Did you undergo revision surgery to treat your complications?
Revision surgery can be a riskier procedure than the original hip replacement surgery, but it may be needed if the patient’s hip replacement wore out or they experienced complications from it. Complications such as blood clots, dislocations, fractures and device loosening can occur as a result of revision surgery.
Calling this number connects you with a Drugwatch.com representative. We will direct you to one of our trusted legal partners for a free case review.
Drugwatch.com's trusted legal partners support the organization's mission to keep people safe from dangerous drugs and medical devices. For more information, visit our partners page.